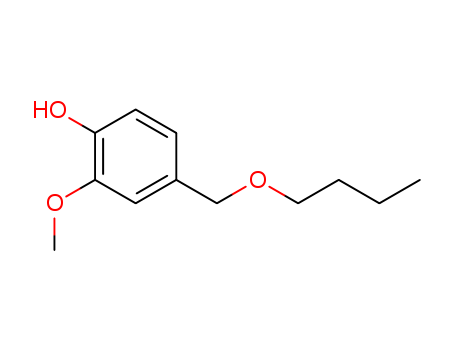
82654-98-6
- Product Name:Vanillyl Butyl Ether
- Molecular Formula:C12H18O3
- Purity:99%
- Molecular Weight:210.273
Product Details;
CasNo: 82654-98-6
Molecular Formula: C12H18O3
Appearance: Colorless transparent liquid
Quality Factory Supply Vanillyl Butyl Ether, Buy 82654-98-6 Fast Shipping
- Molecular Formula:C12H18O3
- Molecular Weight:210.273
- Appearance/Colour:Colorless transparent liquid
- Vapor Pressure:0.000388mmHg at 25°C
- Refractive Index:n20/D 1.516(lit.)
- Boiling Point:307.9 ºC at 760 mmHg
- Flash Point:140 ºC
- PSA:38.69000
- Density:1.048 g/cm3
- LogP:2.71750
- IDLH:888
- IDLH:3796
Vanillyl butyl ether(Cas 82654-98-6) Usage
|
Description |
Vanillyl butyl ether is an ether of monohydroxybenzoic acid. It is added to food products as a flavoring agent. It is also present in cosmetics and personal care products. It has a characteristic trigeminal, burning, hot pepper nature and can be used in spice flavors like pepper, cinnamon and ginger, as well as blends for some baked applications, including cookies. It has some other applications include flavoring for alcoholic and non-alcoholic beverages with herbal, berry, vanilla, cola, ginger ale, root beer, hot chocolate, chai, ice tea and mints. It can also be used as a warming agent. |
|
Chemical Properties |
Vanillyl butyl ether has a weak, vanillic, acidic odor. |
|
Uses |
Vanillyl butyl ether is a compound hypothesized to have warming and vasodilation mechanisms when applied on the skin as a cream. |
|
General Description |
Vanillyl butyl ether is an alkoxy-substituted benzyl derivative mainly used as a flavoring agent. |
|
Biochem/physiol Actions |
Taste at 10-15% |
|
Who Evaluation |
Evaluation year: 2001 |
InChI:InChI=1/C12H18O3/c1-3-4-7-15-9-10-5-6-11(13)12(8-10)14-2/h5-6,8,13H,3-4,7,9H2,1-2H3
82654-98-6 Relevant articles
Synthetic method of vanillyl alcohol ether
-
Paragraph 0044-0046, (2019/07/08)
The invention discloses a synthetic meth...
Technology for preparing vanillyl alcohol ether by using one step method
-
Paragraph 0025; 0026, (2017/03/18)
The invention relates to a technology fo...
Sulfated tungstate as hydroxyl group activator for preparation of benzyl, including p-methoxybenzyl ethers of alcohols and phenols
Katkar, Kamlesh V.,Veer, Sachin D.,Akamanchi, Krishnacharya G.
supporting information, p. 1893 - 1901 (2016/11/25)
Sulfated tungstate was found to be an ef...
Direct synthesis of unsymmetrical ethers from alcohols catalyzed by titanium cation-exchanged montmorillonite
Mitsudome, Takato,Matsuno, Tsuyoshi,Sueoka, Shoichiro,Mizugaki, Tomoo,Jitsukawa, Koichiro,Kaneda, Kiyotomi
supporting information; experimental part, p. 610 - 613 (2012/04/23)
Titanium-exchanged montmorillonite (Ti4+...
82654-98-6 Process route
-
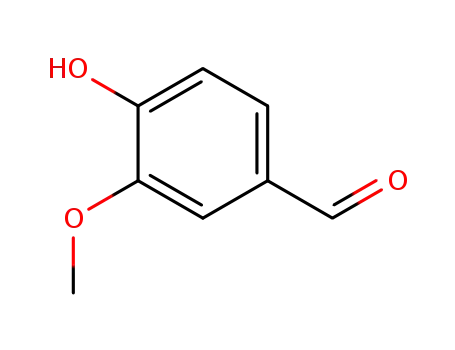
- 121-33-5,8014-42-4
vanillin

-
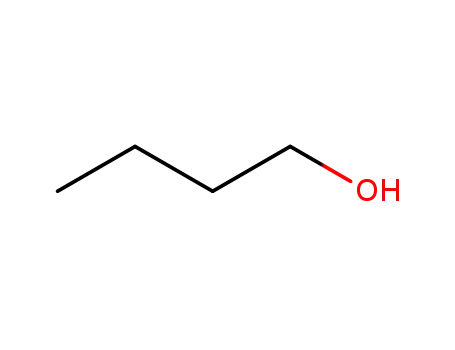
- 71-36-3
butan-1-ol

-
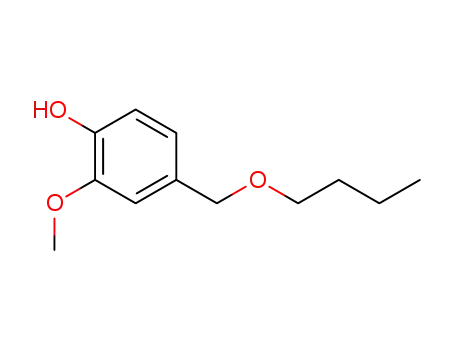
- 82654-98-6
4-(butoxymethyl)-2-methoxyphenol
| Conditions | Yield |
|---|---|
|
With hydrogen; at 20 ℃; for 18h; under 7500.75 Torr; Industrial scale;
|
96% |
|
vanillin; butan-1-ol; With Pb/C; hydrogen; at 60 - 70 ℃; for 8h; under 13501.4 Torr;
With aluminum (III) chloride; at 60 - 70 ℃; for 12h;
|
68.55% |
-
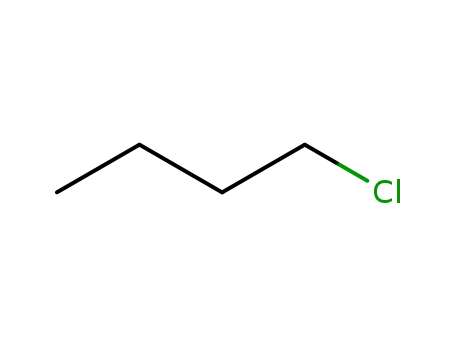
- 109-69-3
n-Butyl chloride

-

- 121-33-5,8014-42-4
vanillin

-

- 82654-98-6
4-(butoxymethyl)-2-methoxyphenol
| Conditions | Yield |
|---|---|
|
With potassium borohydride; In ethyl acetate; at 30 - 40 ℃; for 3h;
|
90.51% |
82654-98-6 Upstream products
-
498-00-0
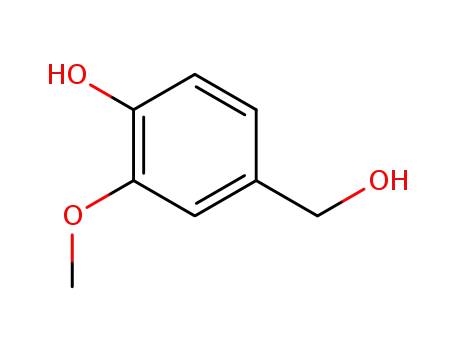
4-hydroxymethyl-2-methoxyphenol
-
71-36-3

butan-1-ol
-
121-33-5

vanillin
-
109-69-3

n-Butyl chloride
Relevant Products
-
Tesofensine powder
CAS:402856-42-2
-
LL-37 amide trifluoroacetate salt
CAS:597562-32-8
-
Carperitide
CAS:89213-87-6