3422-01-3
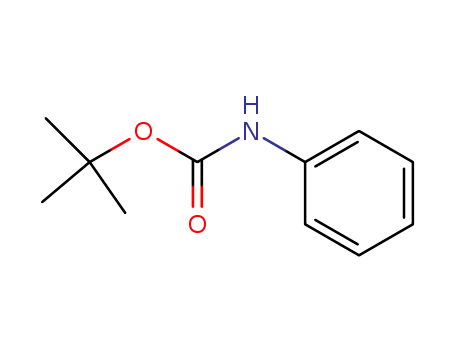
- Product Name:N-BOC-aniline
- Molecular Formula:C11H15NO2
- Purity:99%
- Molecular Weight:193.246
Product Details;
CasNo: 3422-01-3
Molecular Formula: C11H15NO2
High Purity Buy High Grade N-BOC-aniline 3422-01-3 Safe Transportation
- Molecular Formula:C11H15NO2
- Molecular Weight:193.246
- Vapor Pressure:0.0505mmHg at 25°C
- Melting Point:133-137 °C
- Refractive Index:1.541
- Boiling Point:235.3 °C at 760 mmHg
- PKA:13.86±0.70(Predicted)
- Flash Point:96.1 °C
- PSA:38.33000
- Density:1.082 g/cm3
- LogP:3.10660
N-BOC ANILINE(Cas 3422-01-3) Usage
|
Chemical Properties |
Off-White Solid |
|
Uses |
Protected Aniline. |
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 87, p. 1141, 1965 DOI: 10.1021/ja01083a042The Journal of Organic Chemistry, 43, p. 2609, 1978 |
InChI:InChI=1/C11H15NO2/c1-11(2,3)14-10(13)12-9-7-5-4-6-8-9/h4-8H,1-3H3,(H,12,13)
3422-01-3 Relevant articles
Using hydrogen bonding to control carbamate C-N rotamer equilibria
Moraczewski, Alexei L.,Banaszynski, Laura A.,From, Aaron M.,White, Courtney E.,Smith, Bradley D.
, p. 7258 - 7262 (1998)
In chloroform solution, the syn/anti rot...
Radical Hydrodehalogenation of Aryl Halides with H2 Catalyzed by a Phenanthroline-Based PNNP Cobalt(I) Complex
Iizuka, Kosuke,Ishizaka, Yusuke,Jheng, Nai-Yuan,Minami, Yasunori,Naganawa, Yuki,Nakajima, Yumiko,Sekiguchi, Akira
, p. 2320 - 2329 (2022/02/16)
Radical hydrodehalogenation of aryl hali...
Integrating Hydrogen Production and Transfer Hydrogenation with Selenite Promoted Electrooxidation of α-Nitrotoluenes to E-Nitroethenes
Chong, Xiaodan,Liu, Cuibo,Wang, Changhong,Yang, Rong,Zhang, Bin
supporting information, p. 22010 - 22016 (2021/09/02)
Developing an electrochemical carbon-add...
Thiamine hydrochloride as a recyclable organocatalyst for the efficient and chemoselective N-tert-butyloxycarbonylation of amines
Ingale, Ajit P.,Garad, Dnyaneshwar N.,Ukale, Dattatraya,Thorat, Nitin M.,Shinde, Sandeep V.
supporting information, p. 3791 - 3804 (2021/11/04)
Thiamin hydrochloride promoted highly ef...
Ultrasound promoted environmentally benign, highly efficient, and chemoselective N-tert-butyloxycarbonylation of amines by reusable sulfated polyborate
Pise, Ashok S.,Ingale, Ajit P.,Dalvi, Navnath R.
supporting information, p. 3768 - 3780 (2021/10/26)
The sulfated polyborate catalyzed an eff...
3422-01-3 Process route
-
-
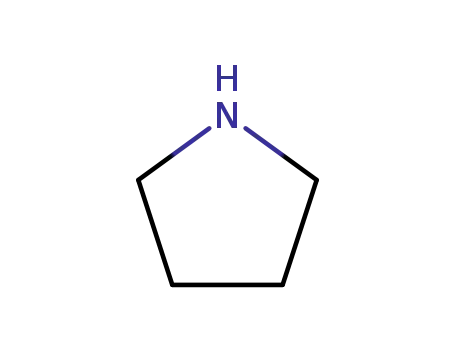
123-75-1
pyrrolidine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
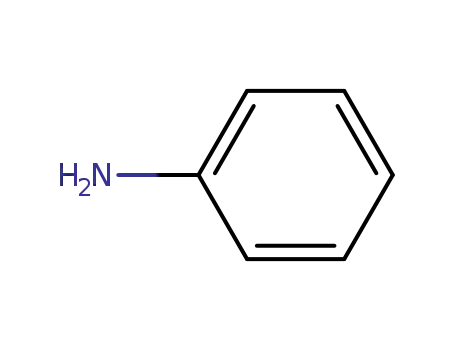
62-53-3
aniline

-
-
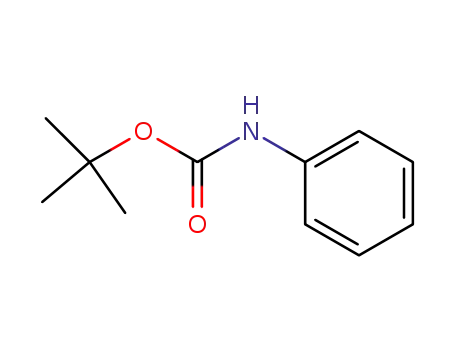
3422-01-3
tert-butyl phenylcarbamate

-
-
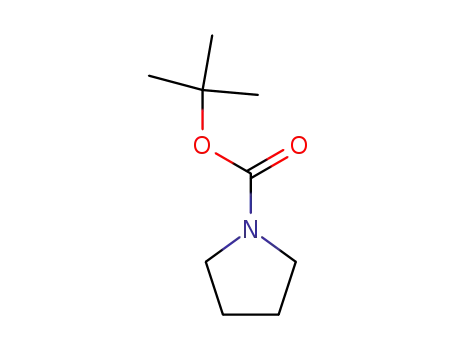
86953-79-9
tert-butyl pyrrolidine-1-carboxylate
| Conditions | Yield |
|---|---|
|
for 0.0333333h;
|
-
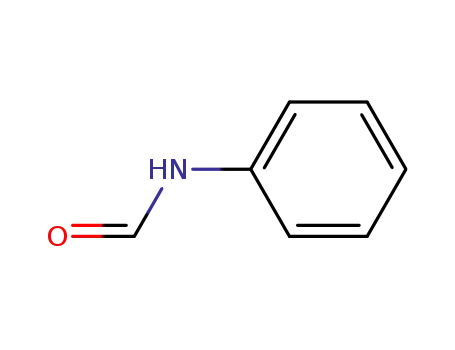
-
103-70-8
Formanilid

-

-
3422-01-3
tert-butyl phenylcarbamate

-
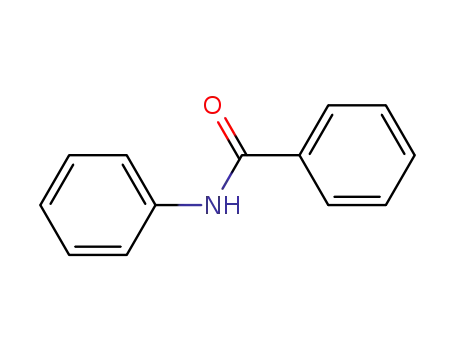
-
93-98-1,5705-51-1
N-phenyl benzoyl amide

-
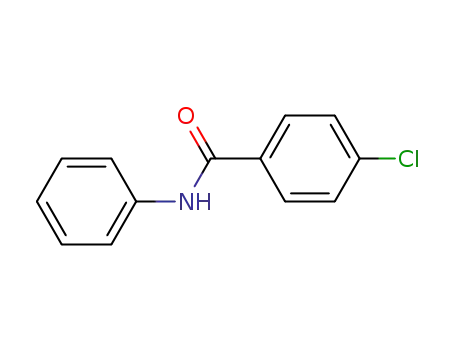
-
6833-15-4
4-chlorobenzanilide

-
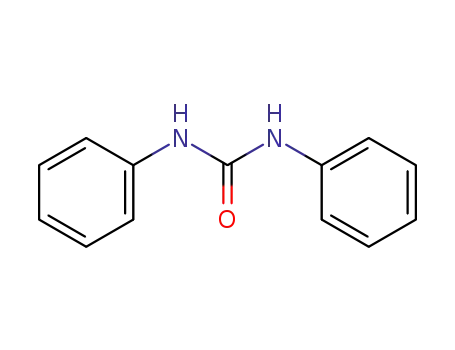
-
102-07-8
bis(diphenyl)urea
| Conditions | Yield |
|---|---|
|
With
di-tert-butyl peroxide;
In
chlorobenzene;
at 110 ℃;
for 48h;
Further byproducts given;
|
4% 3% 20% 25% |
|
With
di-tert-butyl peroxide; chlorobenzene;
at 110 ℃;
for 48h;
Further byproducts given;
|
25% 3% 20% 4% |
3422-01-3 Upstream products
-
103-71-9
phenyl isocyanate
-
75-65-0
tert-butyl alcohol
-
24608-52-4
tert-butyl chloroformate
-
62-53-3

aniline
3422-01-3 Downstream products
-
10264-18-3
N-phenylpentanamide
-
35282-60-1
N-(1,1-dibutylpentyl)benzeneamine
-
144304-01-8
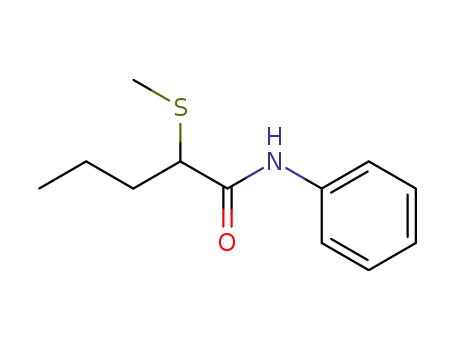
2-(methylthio)-N-phenylpentanamide
-
144303-96-8
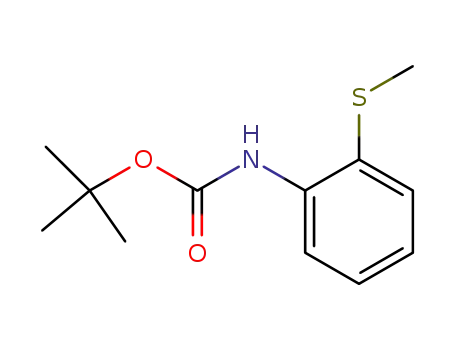
<2-(methylthio)phenyl>carbamic acid 1,1-dimethylethyl ester
Relevant Products
-
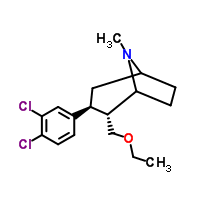
Tesofensine powder
CAS:402856-42-2
-
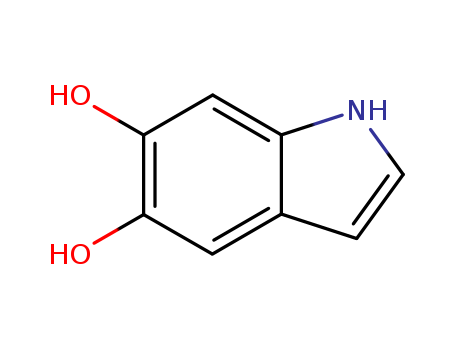
5,6-dihydroxyindole
CAS:3131-52-0
-
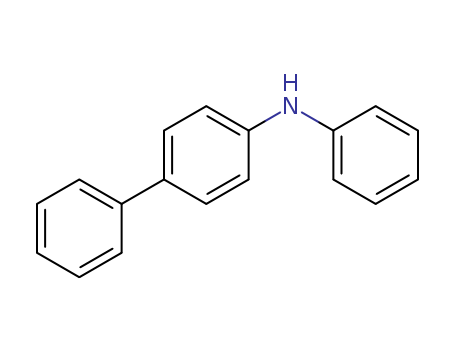
N-Phenyl-4-biphenylamin
CAS:32228-99-2