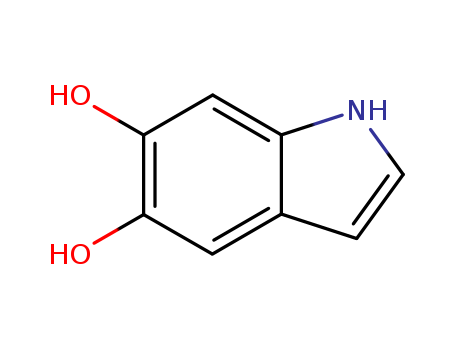
3131-52-0
- Product Name:5,6-dihydroxyindole
- Molecular Formula:C8H7NO2
- Purity:99%
- Molecular Weight:149.149
Product Details;
CasNo: 3131-52-0
Molecular Formula: C8H7NO2
Buy High Quality 99% Pure 5,6-dihydroxyindole 3131-52-0 Efficient Delivery
- Molecular Formula:C8H7NO2
- Molecular Weight:149.149
- Vapor Pressure:2.38E-07mmHg at 25°C
- Melting Point:140 °C (decomp)
- Refractive Index:1.802
- Boiling Point:411.2 °C at 760 mmHg
- PKA:9.81±0.40(Predicted)
- Flash Point:202.5 °C
- PSA:56.25000
- Density:1.51 g/cm3
- LogP:1.57910
5,6-Dihydroxyindole(Cas 3131-52-0) Usage
|
Synthesis Reference(s) |
Synthetic Communications, 15, p. 321, 1985 DOI: 10.1080/00397918508063806 |
InChI:InChI=1/C8H7NO2/c10-7-3-5-1-2-9-6(5)4-8(7)11/h1-4,9-11H
3131-52-0 Relevant articles
In situ insights into the nanoscale deposition of 5,6-dihydroxyindole-based coatings and the implications on the underwater adhesion mechanism of polydopamine coatings
Lyu, Qinghua,Song, Hongyan,Yakovlev, Nikolai L.,Tan, Wui Siew,Chai, Christina L. L.
, p. 27695 - 27702 (2018)
The biomimetic coating polydopamine (PDA...
Short-lived quinonoid species from 5,6-dihydroxyindole dimers en route to eumelanin polymers: Integrated chemical, pulse radiolytic, and quantum mechanical investigation
Pezzella, Alessandro,Panzella, Lucia,Crescenzi, Orlando,Napolitano, Alessandra,Navaratman, Suppiah,Edge, Ruth,Land, Edward J.,Barone, Vincenzo,D'Ischia, Marco
, p. 15490 - 15498 (2006)
The transient species formed by oxidatio...
MECHANISM OF THE REARRANGEMENT OF DOPACHROME TO 5,6-DIHYDROXYINDOLE
Costantini, C.,Crescenzi, O.,Prota, G.
, p. 3849 - 3850 (1991)
Kinetic and isotopic labelling studies p...
Effect of aluminum (III) on the conversion of dopachrome in the melanin synthesis pathway
Di, Junwei,Bi, Shuping
, p. 1689 - 1696 (2003)
The effect of aluminum ions on the kinet...
Effect of stacking and redox state on optical absorption spectra of melanins-comparison of theoretical and experimental results
Stark, Klaus B.,Gallas, James M.,Zajac, Gerry W.,Golab, Joseph T.,Gidanian, Shirley,McIntire, Theresa,Farmer, Patrick J.
, p. 1970 - 1977 (2005)
In this work the effect of aggregation a...
Ultra-low temperature oxidation of 5,6-dihydroxyindole: A novel approach to study synthetic melanogenesis
Hatcher, Lanying Q.,Simon, John D.
, p. 608 - 612 (2008)
The detailed structure of melanin remain...
Detection of melanochromes by MALDI-TOF mass spectrometry
Kroesche, Christoph,Peter, Martin G.
, p. 3947 - 3952 (1996)
Melanin formation from dopamine, DOPA, D...
Evidence for the intermediacy of quinone-methides in the rearrangement of aminochromes to 5,6-dihydroxyindoles
Crescenzi,Costantini,Prota
, p. 6095 - 6096 (1990)
Oxidation of α-methyldopa methyl ester l...
Chemical, pulse radiolysis and density functional studies of a new, labile 5,6-indolequinone and its semiquinone
Pezzella, Alessandro,Crescenzi, Orlando,Natangelo, Anna,Panzella, Lucia,Napolitano, Alessandra,Navaratnam, Suppiah,Edge, Ruth,Land, Edward J.,Barone, Vincenzo,D'Ischia, Marco
, p. 1595 - 1603 (2007)
The chemical and spectroscopic character...
Synthesis and Physical Properties of 5,6-Dihydroxyindole
Murphy, Bryan P.,Schultz, Thomas M.
, p. 2790 - 2791 (1985)
-
Method for preparing 5, 6-dihydroxyindole by using modified ordered mesoporous carbon supported metal catalyst
-
Paragraph 0032; 0039-0041; 0048-0049, (2021/05/01)
The invention belongs to the technical f...
PRODUCTION METHOD FOR DIHYDROXYINDOLES
-
Paragraph 0120-0127, (2020/09/09)
A method for producing dihydroxyindoles ...
Method for efficiently preparing 5,6-dihydroxyindole (by machine translation)
-
Paragraph 0031; 0038-0039; 0040; 0047-0048, (2020/04/29)
The preparation method 5,6 - of the comp...
3131-52-0 Process route
-
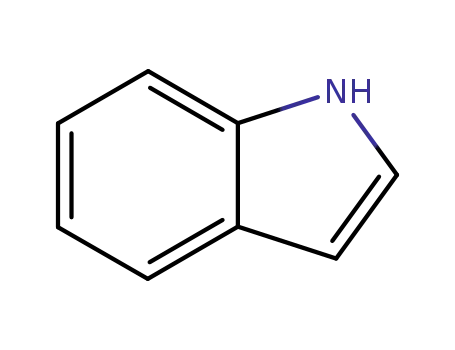
-
120-72-9
indole

-
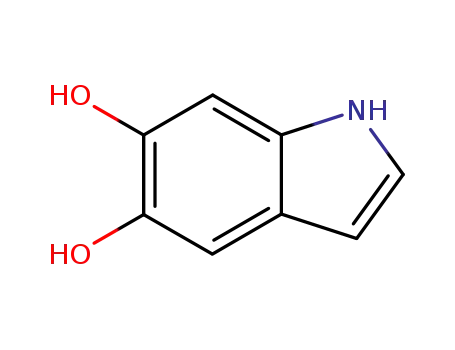
-
3131-52-0
1H-indole-5,6-diol

-
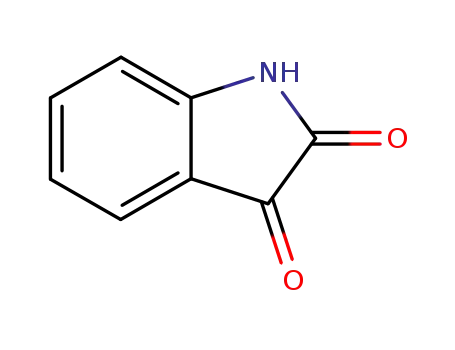
-
91-56-5,1186480-61-4,84788-92-1
indole-2,3-dione

-
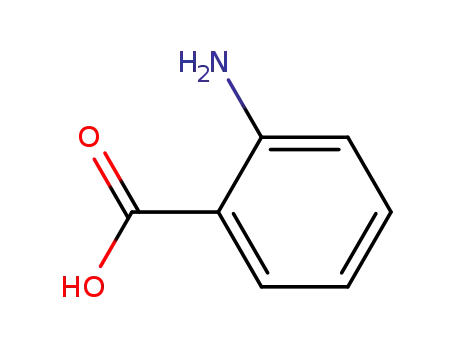
-
118-92-3,159201-01-1,50816-84-7,80206-34-4,104809-47-4
anthranilic acid

-
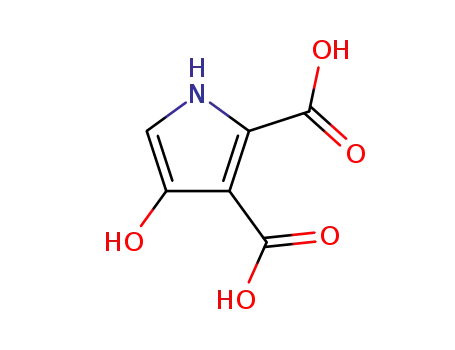
-
3-hydroxy pyrrolle-4,5-dicarboxylic acid

-
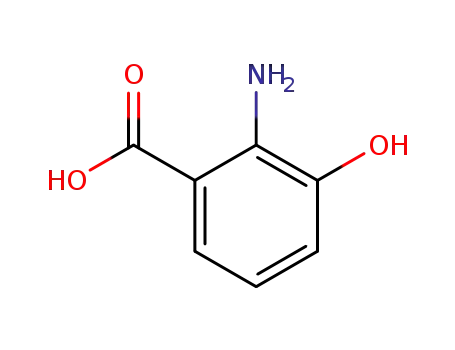
-
548-93-6
3-hydroxyanthranilic acid
| Conditions | Yield |
|---|---|
|
With
phosphate buffer; ethylenediaminetetraacetic acid; iron(II) sulfate; ascorbic acid;
In
water; acetone;
at 37 ℃;
for 2h;
Product distribution;
melanin formation under Udenfriend condition, oxygen atmosphere;
|
-
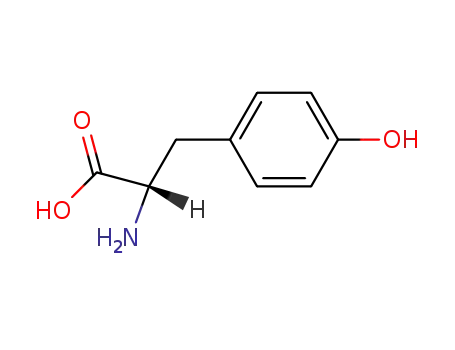
-
60-18-4,18875-48-4,25619-78-7,30704-25-7
L-tyrosine

-

-
3131-52-0
1H-indole-5,6-diol

-
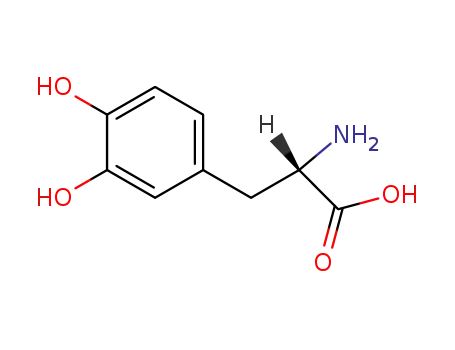
-
59-92-7,90638-38-3
levodopa

-
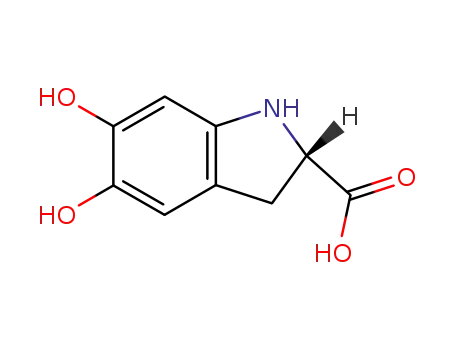
-
18766-67-1
leucodopachromene

-
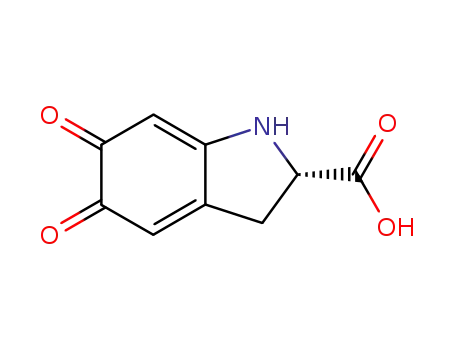
-
3571-34-4,89762-39-0
(2S)-dopachrome
| Conditions | Yield |
|---|---|
|
With
water;
mushroom tyrosinase;
Rate constant;
phosphat buffer, ph 6.8;
|
3131-52-0 Upstream products
-
38411-80-2
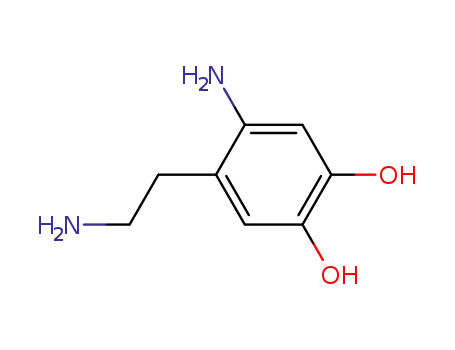
1-(2-amino-4,5-dihydroxyphenyl)-2-ethylamine
-
15069-79-1
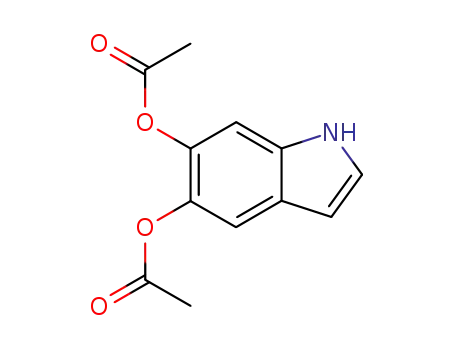
5,6-diacetoxyindole
-
636-00-0
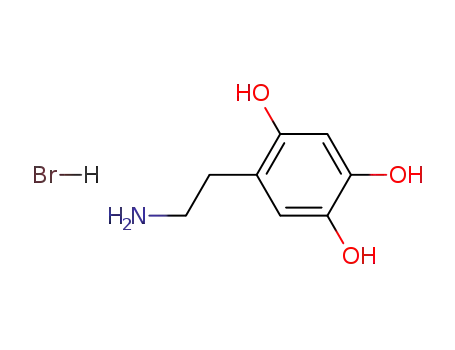
6-hydroxydopamine hydrobromide
-
51-61-6
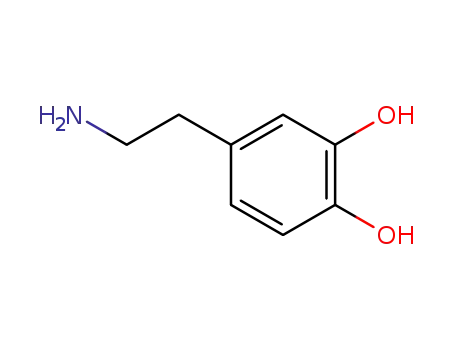
dopamine
3131-52-0 Downstream products
-
15069-79-1

5,6-diacetoxyindole
-
1134783-55-3
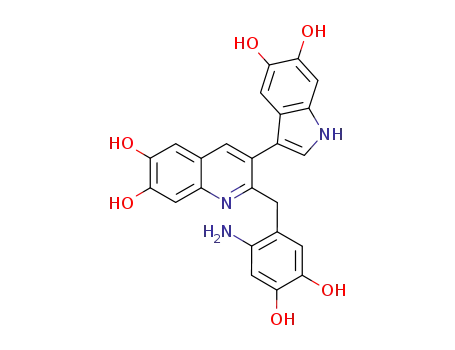
2-(2-amino-4,5-dihydroxybenzyl)-6,7-dihydroxy-3-(5,6-dihydroxyindol-3-yl)quinoline
-
1134783-53-1
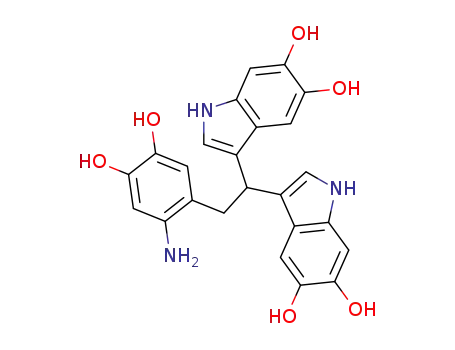
C24H21N3O6
-
124673-68-3
indole-5,6-quinone
Relevant Products
-
Tesofensine powder
CAS:402856-42-2
-
Bemotrizinol
CAS:187393-00-6
-
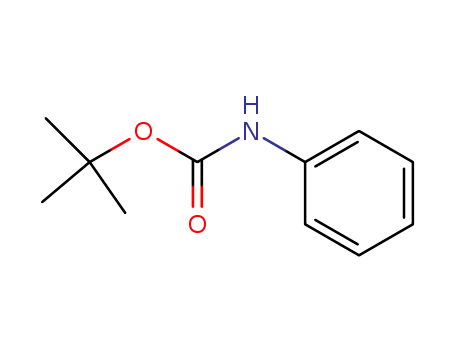
N-BOC-aniline
CAS:3422-01-3