133-37-9
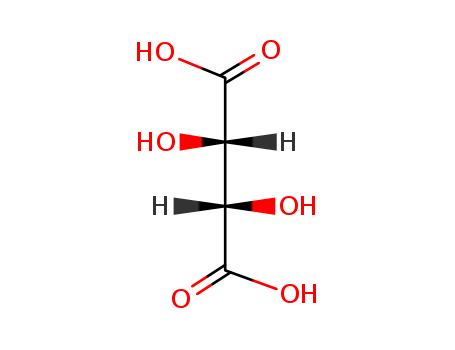
- Product Name:DL-Tartaric Acid
- Molecular Formula:C4H6O6
- Purity:99%
- Molecular Weight:150.088
Product Details;
CasNo: 133-37-9
Molecular Formula: C4H6O6
Appearance: white crystalline powder
Quality Manufacturer Supply Top Purity 99% DL-Tartaric Acid 133-37-9 Efficient Transportation
- Molecular Formula:C4H6O6
- Molecular Weight:150.088
- Appearance/Colour:white crystalline powder
- Vapor Pressure:4.93E-08mmHg at 25°C
- Melting Point:210-212 °C(lit.)
- Refractive Index:1.585
- Boiling Point:399.3 °C at 760 mmHg
- PKA:3.03, 4.37(at 25℃)
- Flash Point:209.4 °C
- PSA:115.06000
- Density:1.886 g/cm3
- LogP:-2.12260
- IDLH:621
DL-Tartaric acid(Cas 133-37-9) Usage
|
DESCRIPTION |
Colourless or translucent crystals, or a white crystalline powder; odourless |
|
USES |
Synergist for antioxidants, acid, emulsifier, sequestrant, flavouring agent. Tartaric acid and its derivatives have a plethora of uses in the field of pharmaceuticals. It has been used in the production of effervescent salts, in combination with citric acid, in order to improve the taste of oral medications. The potassium antimonyl derivative of the acid known as tartar emetic is included, in small doses, in cough syrup as an expectorant. Further, it is used as lubricant and grease. It is mixed with sodium bicarbonate and used as a leavening agent in food preparation. In the pharmaceutical industry, it is utilized in the preparation of tartar emetic, which is used in cough syrup as an expectorant. |
|
Solubility |
Freely soluble in water; sparingly soluble in ethanol |
|
Melting range |
200 - 206o with decomposition when heated rapidly in a sealed capillarytube |
|
substances |
N potassium permanganate while keeping the solution at 20o . The colour of the solution does not disappear within 3 min. |
|
METHOD OF ASSAY |
Weigh accurately about 2 g of the dried sample, dissolve it in 40 ml of water, add phenolphthalein TS, and titrate with 1 N sodium hydroxide. Each ml of 1 N sodium hydroxide is equivalent to 75.04 mg of C4H6O6. |
|
Chemical Properties |
Tartaric acid, HOOC(CHOH)2COOH, is a water- and alcohol-soluble colorless crystalline solid with an acid taste and a melting temperature of 170°C (338 OF). It is also known as dihydroxy succinic acid. Tartaric acid is used as a chemical intermediate and a sequestrant,as well as in tanning, effervescent beverages, baking powder, ceramics, photography, textile processing,mirror silvering,and metal coloring. |
|
Occurrence |
d-Tartaric acid occurs in many fruits or other parts of the plant, free or combined with potassium, calcium or magnesium. It is also reported found in raw, lean fish, white wine, red wine and port wine. |
|
Preparation |
The tartrates used in commerce are obtained as a by-product of wine manufacture and have the L(+) configuration. Produced from argols or wine lees, which are formed in the manufacture of wine by extracting the potassium acid tartrate, transforming this into the calcium salt and then acidifying with dilute sulfuric acid; also by oxidation of d-glucose with nitric acid. The dl-tartaric acid is obtained by boiling the d-tartaric acid with an aqueous solution of NaOH or by oxidation of fumaric acid. The l- and the meso-tartaric acid are also known, but are less important. |
|
Who Evaluation |
Evaluation year: 1977 |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C4H6O6/c5-1(3(7)8)2(6)4(9)10/h1-2,5-6H,(H,7,8)(H,9,10)/t1-,2-/m1/s1
133-37-9 Relevant articles
Supramolecular metathesis: Co-former exchange in co-crystals of pyrazine with (R,R)-, (S,S)-, (R,S)- and (S,S/R,R)-tartaric acid
Braga, Dario,Grepioni, Fabrizia,Lampronti, Giulio I.
, p. 3122 - 3124 (2011)
Co-crystals of dextro-(R,R), levo-(S,S),...
Decorated single-enantiomer phosphoramide-based silica/magnetic nanocomposites for direct enantioseparation
Karimi Ahmadabad, Fatemeh,Pourayoubi, Mehrdad,Bakhshi, Hadi
, p. 27147 - 27156 (2019)
The nano-composites Fe3O4SiO2(-O3Si[(CH2...
4-AcNH-Tempo-catalyzed oxidation of aldoses to aldaric acids using chlorine or bromine as terminal oxidants
Merbouh, Nabyl,Bobbitt, James M.,Brueckner, Christian
, p. 64 - 78 (2007/10/03)
The 4-AcNH-TEMPO-catalyzed oxidation of ...
133-37-9 Process route
-
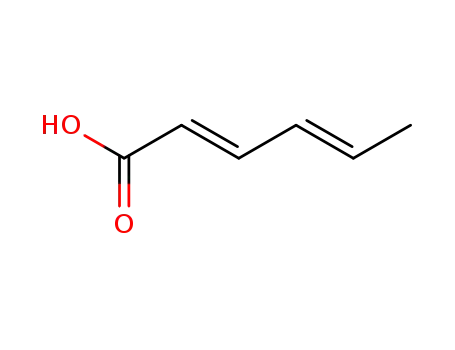
- 110-44-1,91751-55-2
sorbic Acid

-
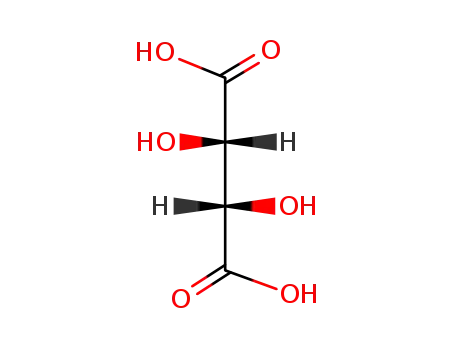
- 133-37-9,138508-61-9
DL-tartaric acid
| Conditions | Yield |
|---|---|
|
With potassium permanganate; at 0 ℃;
|
-

- 74-90-8
hydrogen cyanide

-
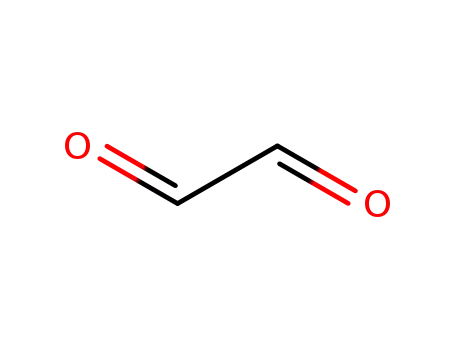
- 131543-46-9,107-22-2
Glyoxal

-

- 133-37-9,138508-61-9
DL-tartaric acid
| Conditions | Yield |
|---|---|
|
With hydrogenchloride;
|
133-37-9 Upstream products
-
110-44-1

sorbic Acid
-
74-90-8

hydrogen cyanide
-
131543-46-9

Glyoxal
-
69-65-8
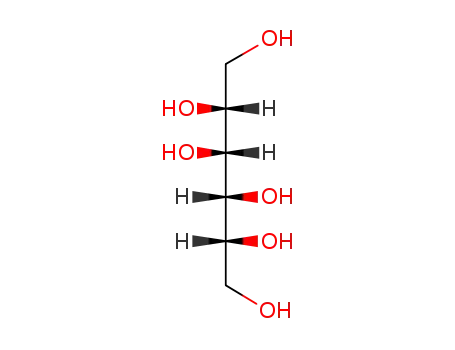
mannitol
133-37-9 Downstream products
-
608-89-9
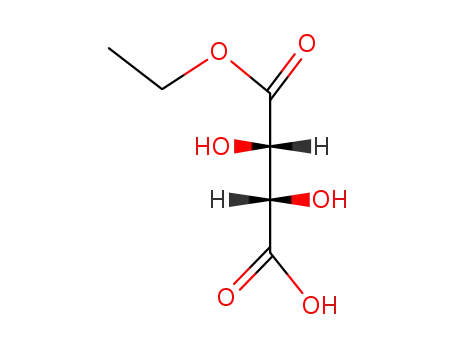
DL-tartaric acid monoethyl ester
-
57968-71-5
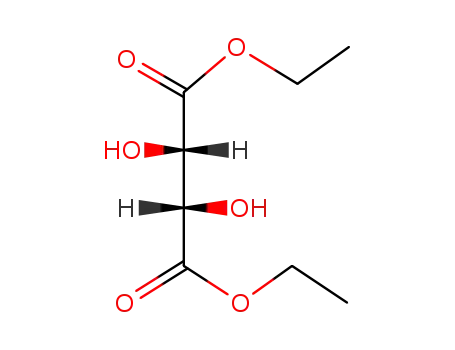
(+/-)-diethyl tartrate
-
4054-82-4
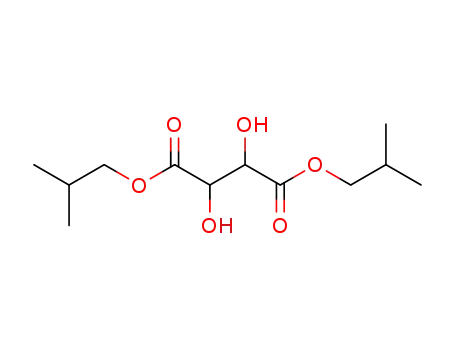
tartaric acid diisobutyl ester
-
72842-25-2
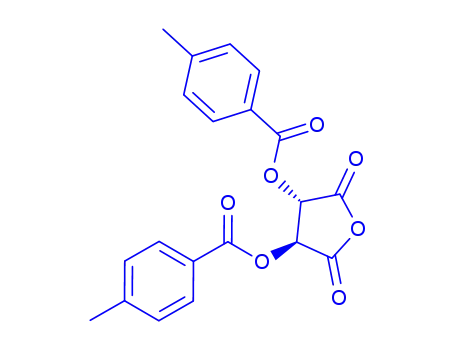
di-O-p-toluoyl-DL-threaric acid-anhydride
Relevant Products
-
Tesofensine powder
CAS:402856-42-2
-
Elamipretide
CAS:736992-21-5
-
PF-04971729
CAS:1210344-57-2