1210344-57-2
- Product Name:PF-04971729
- Molecular Formula:C22H25ClO7
- Purity:99%
- Molecular Weight:436.889
Product Details;
CasNo: 1210344-57-2
Molecular Formula: C22H25ClO7
Quality Factory Supply Top Purity 99% PF-04971729 1210344-57-2 On Stock
- Molecular Formula:C22H25ClO7
- Molecular Weight:436.889
- Boiling Point:630.5±55.0 °C(Predicted)
- PKA:12.95±0.70(Predicted)
- PSA:108.61000
- Density:1.455
- LogP:1.35650
PF-04971729 (Cas 1210344-57-2) Usage
|
Description |
PF-04971729 is an inhibitor of sodium-glucose cotransporter 2 (SGLT2; IC50 = 0.927 nM for the human enzyme). |
|
Uses |
PF-04971729 is a sodium-dependent glucose cotransporter-2 (SGLT2) inhibitor used to treat type II diabetes mellitus. |
1210344-57-2 Relevant articles
AN EFFICIENT PROCESS FOR THE PREPARATION OF ERTUGLIFLOZIN L-PYROGLUTAMIC ACID AND INTERMEDIATES THEREOF
-
, (2021/07/10)
The present invention relates to an effi...
AN IMPROVED PURIFICATION PROCESS FOR THE PREPARATION OF ERTUGLIFLOZIN AND ERTUGLIFLOZIN L-PYROGLUTAMIC ACID CO-CRYSTAL
-
Page/Page column 18; 17, (2022/01/04)
The present invention relates to an impr...
AMORPHOUS ERTUGLIFLOZIN AND PROCESS FOR ITS PREPARATION
-
, (2020/12/13)
An amorphous form of ertugliflozin and p...
A NOVEL PROCESS FOR THE PREPARATION OF SGLT-2 INHIBITORS
-
, (2020/01/24)
The present invention relates to a novel...
1210344-57-2 Process route
-
![[(1S,2S,3S,4R,5S)-2,3,4-tribenzyloxy-5-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-6,8-dioxabicyclo[3.2.1]octane-1-yl]methanol](/upload/2023/11/cd5b0d9c-e474-4b38-8880-75d529568a9f.png)
-
[(1S,2S,3S,4R,5S)-2,3,4-tribenzyloxy-5-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-6,8-dioxabicyclo[3.2.1]octane-1-yl]methanol

-
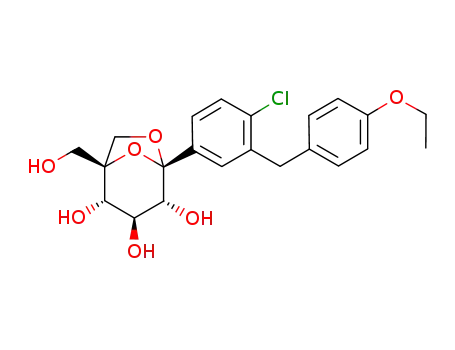
- 1210344-57-2
Ertugliflozin
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; palladium 10% on activated carbon; hydrogen; In tetrahydrofuran; methanol; water; at 20 ℃; for 3h;
|
89% |
|
With palladium on activated charcoal; hydrogen; In ethanol; ethyl acetate; at 20 ℃; for 6h;
|
80% |
|
With palladium on activated charcoal; hydrogen; In tetrahydrofuran; methanol; 1,2-dichloro-benzene; at 25 - 30 ℃; for 6h; under 1500.15 - 2625.26 Torr;
|
13.5 g |
-
![[(3S,4S,5R,6S)-3,4,5-tribenzyloxy-6-[4-chloro-3-[(4-ethoxy-phenyl)methyl]phenyl]-2-(hydroxymethyl)-6-methoxy-tetrahydropyran-2-yl]methanol](/upload/2023/11/9244d42e-dd73-4b20-9d20-7d938e526ae3.png)
-
[(3S,4S,5R,6S)-3,4,5-tribenzyloxy-6-[4-chloro-3-[(4-ethoxy-phenyl)methyl]phenyl]-2-(hydroxymethyl)-6-methoxy-tetrahydropyran-2-yl]methanol

-

- 1210344-57-2
Ertugliflozin
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; palladium 10% on activated carbon; hydrogen; In tetrahydrofuran; methanol; water; at 30 ℃; for 8h;
|
91.4% |
1210344-57-2 Upstream products
-
1210763-25-9
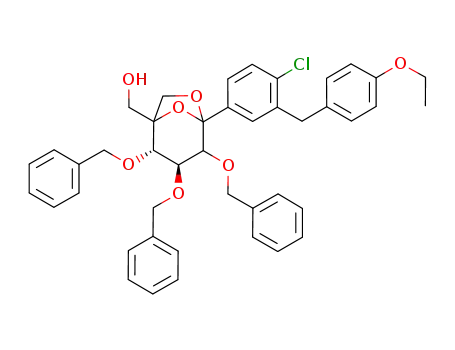
{(2S,3S)-3,4,5-tris-benzyloxy-5-[4-chloro-3-(4-ethoxy-benzyl)-phenyl]-6,8-dioxa-bicyclo[3.2.1]oct-1-yl}-methanol
-
1233482-00-2
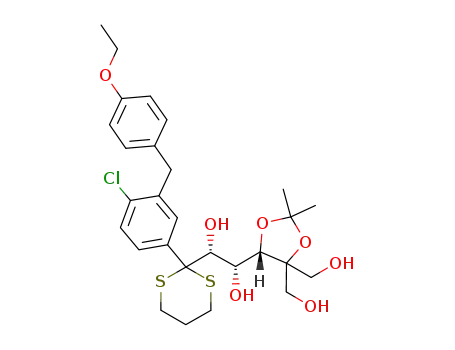
C28H37ClO7S2
-
1629222-50-9
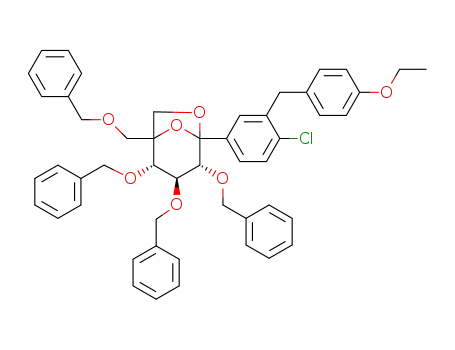
(2S,3S,4R)-2,3,4-tris(benzyloxy)-1-((benzyloxy)-methyl)-5-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6,8-dioxabicyclo[3.2.1]octane
-
1298086-18-6
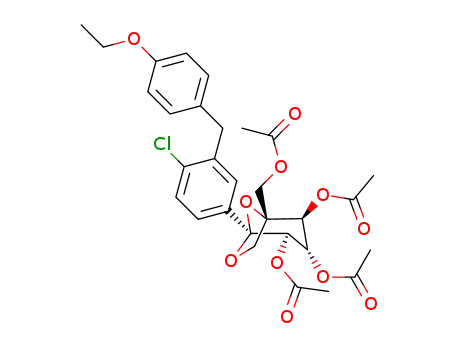
(1R,2S,3S,4R,5S)-1-(acetoxymethyl)-5-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6,8-dioxabicyclo[3.2.1]octane-2,3,4-triyl triacetate
1210344-57-2 Downstream products
-
1292821-53-4
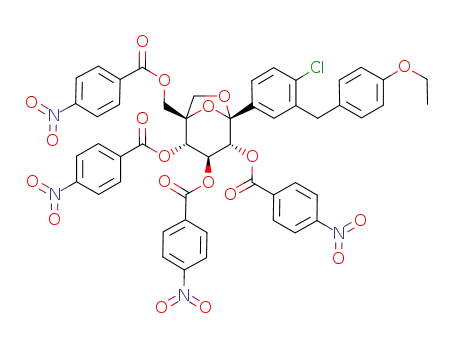
C50H37ClN4O19
-
1298086-20-0
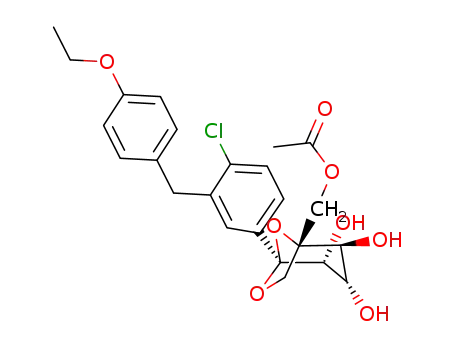
((1R,2S,3S,4R,5S)-5-(4-chloro-3-(4-ethoxybenzyl)-phenyl)-2,3,4-trihydroxy-6,8-dioxabicyclo[3.2.1]octan-1-yl)methyl acetate
-
1298086-18-6

(1R,2S,3S,4R,5S)-1-(acetoxymethyl)-5-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6,8-dioxabicyclo[3.2.1]octane-2,3,4-triyl triacetate
-
1210344-83-4
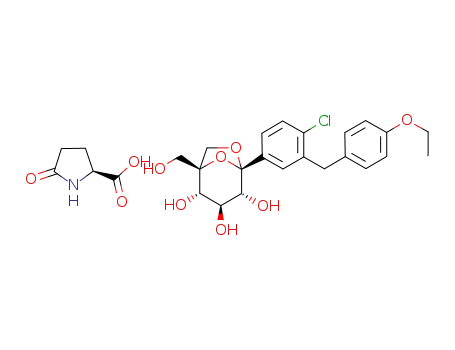
(1S,2S,3S,4R,5S)-5-(4-chloro-3-(4-ethoxybenzyl)phenyl)-1-(hydroxymethyl)-6,8-dioxabicyclo[3.2.1]octane-2,3,4-triol (1:1) compound with (S)-5-oxopyrrolidine-2-carboxylic acid
Relevant Products
-
Tesofensine powder
CAS:402856-42-2
-
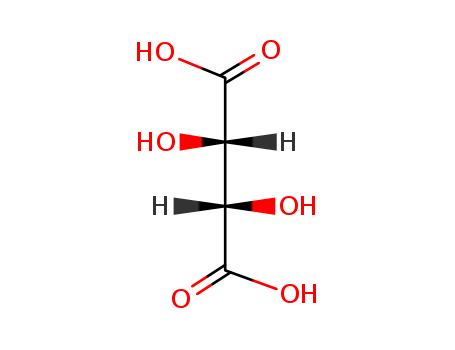
DL-Tartaric Acid
CAS:133-37-9
-
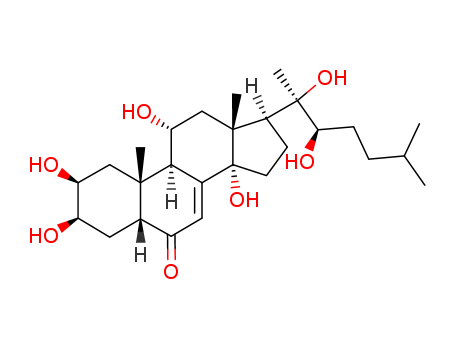
Ajugasterone C
CAS:23044-80-6