6218-29-7
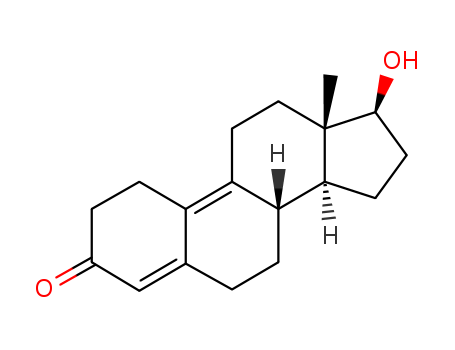
- Product Name:9(10)-Dehydronandrolone
- Molecular Formula:C18H24O2
- Purity:99%
- Molecular Weight:272.387
Product Details;
CasNo: 6218-29-7
Molecular Formula: C18H24O2
Buy Quality Manufacturer Supply 9(10)-Dehydronandrolone 6218-29-7 Lowest Price
- Molecular Formula:C18H24O2
- Molecular Weight:272.387
- Melting Point:190°C(lit.)
- Refractive Index:1.585
- Boiling Point:466.823 °C at 760 mmHg
- PKA:15.00±0.40(Predicted)
- Flash Point:198.572 °C
- PSA:37.30000
- Density:1.17g/cm3
- LogP:3.16320
9(10)-Dehydronandrolone(Cas 6218-29-7) Usage
|
Chemical Properties |
Off-white Solid |
|
Uses |
Gestagenic Testosterone (T155000) derivative. A steroid ligand for the cytosol androgen receptor (AR) of rat prostate. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h10,15-17,20H,2-9H2,1H3/t15?,16?,17?,18-/m0/s1
6218-29-7 Relevant articles
Preparation method of estra-4,9-diene-3,17-dione
-
Paragraph 0058-0059, (2018/05/30)
The invention relates to the technical f...
Regio- and stereoselective reduction of 17-oxosteroids to 17β-hydroxysteroids by a yeast strain Zygowilliopsis sp. WY7905
Liu, Yuanyuan,Wang, Yu,Chen, Xi,Wu, Qiaqing,Wang, Min,Zhu, Dunming,Ma, Yanhe
, p. 17 - 24 (2016/12/22)
The reduction of 17-oxosteroids to 17β-h...
A simple method for the small scale synthesis and solid-phase extraction purification of steroid sulfates
Waller, Christopher C.,McLeod, Malcolm D.
supporting information, p. 74 - 80 (2015/02/19)
Steroid sulfates are a major class of st...
11β-alkyl-Δ9-19-nortestosterone derivatives: High-affinity ligands and potent partial agonists of the androgen receptor
Muddana, Smita S.,Price, Aimee M.,MacBride, Megan M.,Peterson, Blake R.
, p. 4985 - 4988 (2007/10/03)
We report the synthesis of novel steroid...
6218-29-7 Process route
-
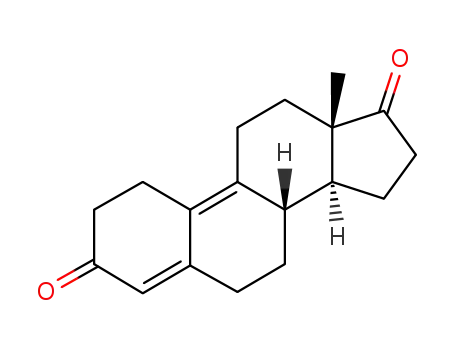
-
5173-46-6
4,9-Androstadiene-3,17-dione

-
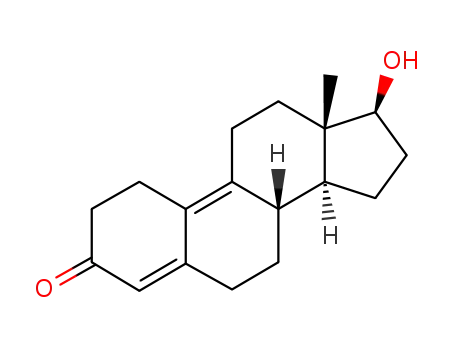
-
6218-29-7
17β-hydroxyestra-4,9-dien-3-one
| Conditions | Yield |
|---|---|
|
With
methanol; sodium tetrahydroborate;
In
dichloromethane;
at -78 ℃;
for 5h;
|
87% |
|
With
zygowilliopsis sp. WY7905;
In
aq. phosphate buffer;
at 30 ℃;
for 24h;
pH=8;
stereoselective reaction;
Enzymatic reaction;
|
61% |
|
With
sodium borohydrid; acetic acid;
In
tetrahydrofuran; methanol;
|
|
|
With
sodium borohydrid; acetic acid;
In
tetrahydrofuran; methanol;
|
-
-
C24H38O2Si

-

-
6218-29-7
17β-hydroxyestra-4,9-dien-3-one
| Conditions | Yield |
|---|---|
|
With
tetrabutyl ammonium fluoride;
In
tetrahydrofuran;
at 20 ℃;
for 0.5h;
|
6218-29-7 Upstream products
-
1089-78-7
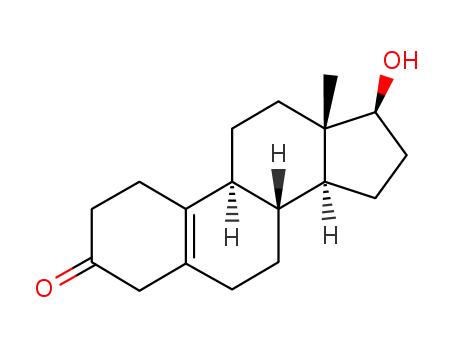
17β-hydroxy-estr-5(10)-en-3-one
-
53303-91-6
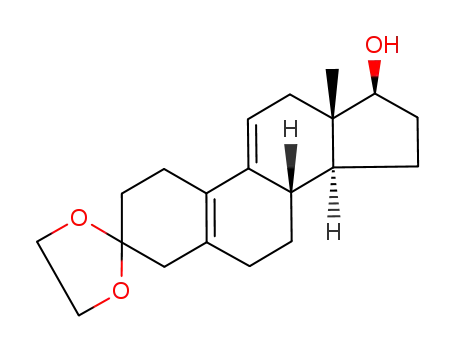
(8S,13S,14S,17S)-13-methyl-1,2,4,6,7,8,12,13,14,15,16,17-dodecahydrospiro[cyclopenta[a]phenanthrene-3,2'-[1,3]dioxolan]-17-ol
-
64-19-7
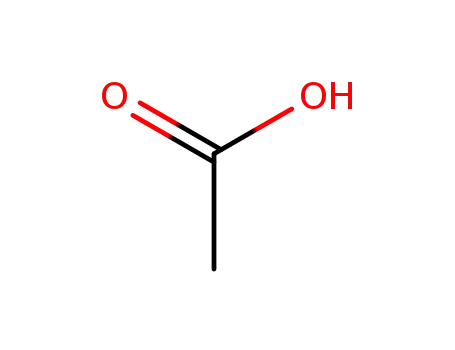
acetic acid
-
1091-93-6
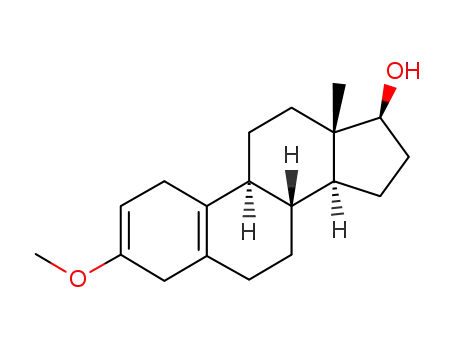
3-methoxy-17-hydroxyestra-2,5(10)-diene
6218-29-7 Downstream products
-
5173-46-6

4,9-Androstadiene-3,17-dione
-
65928-58-7
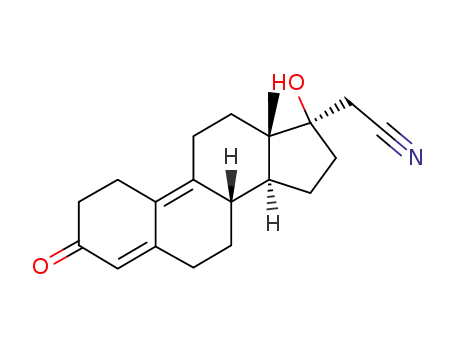
Dienogest
-
57780-81-1
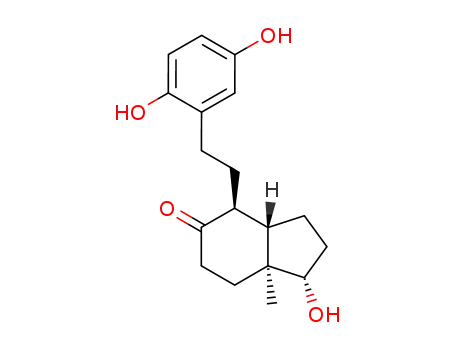
(1S,3aS,4S,7aS)-4-[2-(2,5-Dihydroxy-phenyl)-ethyl]-1-hydroxy-7a-methyl-octahydro-inden-5-one
-
57780-85-5
1,4,17β-Tribenzoyloxy-1,3,5(10),9(11)-estratetraen
Relevant Products
-
Tesofensine powder
CAS:402856-42-2
-
Stearoyl Vanillylamide
CAS:58493-50-8
-
4-chloro-17a-methyl-androst-1,4-diene-3,17b-diol
CAS:1338221-84-3