58493-50-8
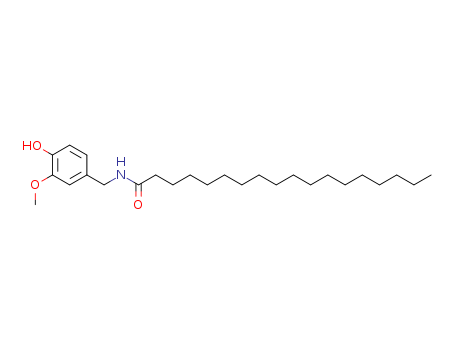
- Product Name:Stearoyl Vanillylamide
- Molecular Formula:C26H45NO3
- Purity:99%
- Molecular Weight:419.648
Product Details;
CasNo: 58493-50-8
Molecular Formula: C26H45NO3
Export Quality Factory Supply Stearoyl Vanillylamide 58493-50-8 Efficient Shipping
- Molecular Formula:C26H45NO3
- Molecular Weight:419.648
- Vapor Pressure:2.46E-14mmHg at 25°C
- Melting Point:94.5-95 °C
- Refractive Index:1.499
- Boiling Point:586.1 °C at 760 mmHg
- PKA:9.76±0.20(Predicted)
- Flash Point:308.3 °C
- PSA:62.05000
- Density:0.973 g/cm3
- LogP:8.11880
Octadecanamide, N-((4-hydroxy-3-methoxyphenyl)methyl)-(Cas 58493-50-8) Usage
InChI:InChI=1/C26H45NO3/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-26(29)27-22-23-19-20-24(28)25(21-23)30-2/h19-21,28H,3-18,22H2,1-2H3,(H,27,29)
58493-50-8 Relevant articles
Highly efficient synthesis of capsaicin analogues by condensation of vanillylamine and acyl chlorides in a biphase H2O/CHCl3 system
Wang, Bo,Yang, Fan,Shan, Yi-Fan,Qiu, Wen-Wei,Tang, Jie
supporting information; experimental part, p. 5409 - 5412 (2009/10/17)
Highly efficient synthesis of capsaicin ...
Lipophilicity of capsaicinoids and capsinoids influences the multiple activation process of rat TRPV1
Morita, Akihito,Iwasaki, Yusaku,Kobata, Kenji,Iida, Tohko,Higashi, Tomohiro,Oda, Kyoko,Suzuki, Asami,Narukawa, Masataka,Sasakuma, Shiho,Yokogoshi, Hidehiko,Yazawa, Susumu,Tominaga, Makoto,Watanabe, Tatsuo
, p. 2303 - 2310 (2007/10/03)
Analogs of capsaicin, such as capsaicino...
N-acylvanillamides: Development of an expeditious synthesis and discovery of new acyl templates for powerful activation of the vanilloid receptor
Appendino, Giovanni,Minassi, Alberto,Morello, Aniello Schiano,De Petrocellis, Luciano,Di Marzo, Vincenzo
, p. 3739 - 3745 (2007/10/03)
A simple and general synthesis of vanill...
Vanilloids. 1. Analogs of Capsaicin with Antinociceptive and Antiinflammatory Activity
Janusz, John M.,Buckwalter, Brian L.,Young, Patricia A.,LaHann, Thomas R.,Farmer, Ralph W.,et al.
, p. 2595 - 2604 (2007/10/02)
As part of a program to establish struct...
58493-50-8 Process route
-
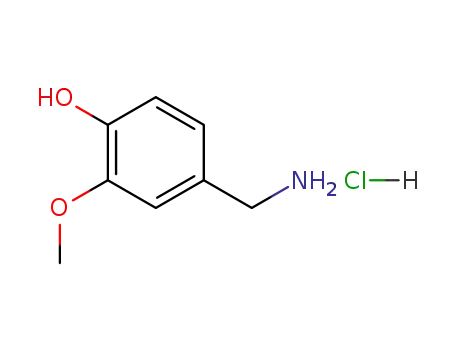
-
7149-10-2
vanillylamine hydrochloride

-
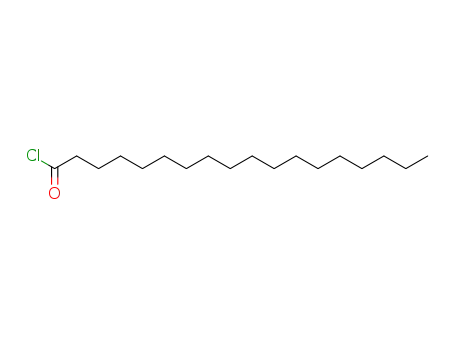
-
112-76-5
Stearoyl chloride

-
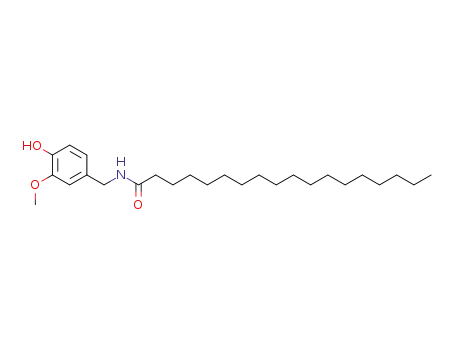
-
58493-50-8
N-Vanillyloctadecanamide
| Conditions | Yield |
|---|---|
|
vanillylamine hydrochloride;
With
sodium hydrogencarbonate;
In
water;
at 20 ℃;
for 0.5h;
Stearoyl chloride;
In
chloroform; water;
at 20 ℃;
for 0.5h;
|
96% |
|
With
pyridine;
|
-

-
7149-10-2
vanillylamine hydrochloride

-
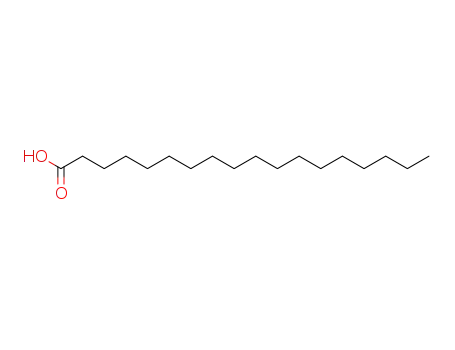
-
57-11-4
stearic acid

-

-
58493-50-8
N-Vanillyloctadecanamide
| Conditions | Yield |
|---|---|
|
With
diethyl cyanophosphonate; triethylamine;
In
tetrahydrofuran;
|
76% |
58493-50-8 Upstream products
-
1196-92-5
Vanillylamin
-
112-76-5
Stearoyl chloride
-
7149-10-2

vanillylamine hydrochloride
-
57-11-4
stearic acid
Relevant Products
-
Tesofensine powder
CAS:402856-42-2
-
Estra-4,9-diene-3,17-dione
CAS:5173-46-6
-
9(10)-Dehydronandrolone
CAS:6218-29-7