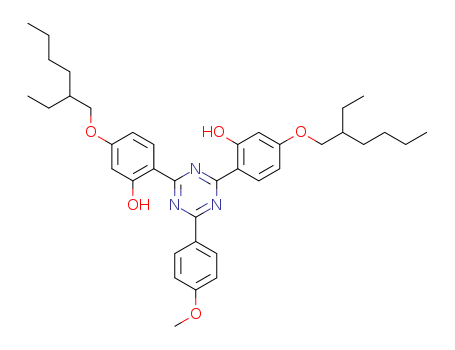
1825352-65-5
- Product Name:Risdiplam
- Molecular Formula:
- Purity:99%
- Molecular Weight:401.471
Product Details;
CasNo: 1825352-65-5
Buy Reliable Quality High Purity 99% Risdiplam 1825352-65-5 Safe Delivery
- Molecular Formula:C22H23N7O
- Molecular Weight:401.471
- Density:1.50±0.1 g/cm3(Predicted)
1825352-65-5 Relevant articles
PROCESS FOR THE PREPRATION OF 7-(4,7-DIAZASPIRO[2.5]OCTAN-7-YL)-2-(2,8-DIMETHYLIMIDAZO[1,2-B]PYRIDAZIN-6-YL)PYRIDO[1,2-A]PYRIMIDIN-4-ONE DERIVATIVES
-
, (2019/04/16)
The present invention relates to a proce...
COMPOUNDS FOR TREATING SPINAL MUSCULAR ATROPHY
-
, (2019/10/29)
The present invention provides compounds...
Discovery of Risdiplam, a Selective Survival of Motor Neuron-2 (SMN2) Gene Splicing Modifier for the Treatment of Spinal Muscular Atrophy (SMA)
Ratni, Hasane,Ebeling, Martin,Baird, John,Bendels, Stefanie,Bylund, Johan,Chen, Karen S.,Denk, Nora,Feng, Zhihua,Green, Luke,Guerard, Melanie,Jablonski, Philippe,Jacobsen, Bjoern,Khwaja, Omar,Kletzl, Heidemarie,Ko, Chien-Ping,Kustermann, Stefan,Marquet, Anne,Metzger, Friedrich,Mueller, Barbara,Naryshkin, Nikolai A.,Paushkin, Sergey V.,Pinard, Emmanuel,Poirier, Agnès,Reutlinger, Michael,Weetall, Marla,Zeller, Andreas,Zhao, Xin,Mueller, Lutz
, p. 6501 - 6517 (2018/08/17)
SMA is an inherited disease that leads t...
COMPOUNDS FOR TREATING AMYOTROPHIC LATERAL SCLEROSIS
-
, (2017/06/01)
The present invention provides compounds...
1825352-65-5 Process route
-
![6-chloro-2,8-dimethyl-imidazo[1,2-b]pyridazine](/upload/2023/11/5e6530b6-473a-4a0e-ad7d-514bfdaa8444.png)
-
17412-23-6
6-chloro-2,8-dimethyl-imidazo[1,2-b]pyridazine

-
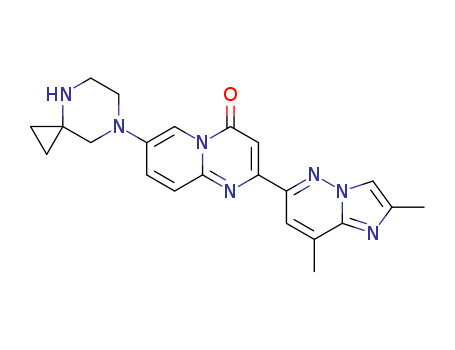
![7-(4,7-diazaspiro[2.5]octan-7-yl)-2-(2,8-dimethylimidazo[l,2- b]pyridazin-6-yl)-4H-pyrido[l,2-a]pyrimidin-4-one](/upload/2023/11/4843d8bb-6708-4bb2-816a-d69c38a758e8.png)
-
1825352-65-5
7-(4,7-diazaspiro[2.5]octan-7-yl)-2-(2,8-dimethylimidazo[l,2- b]pyridazin-6-yl)-4H-pyrido[l,2-a]pyrimidin-4-one
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate / 1,4-dioxane / 15 h / 110 °C / Inert atmosphere
2: tetrakis(triphenylphosphine) palladium(0); potassium carbonate / water; acetonitrile / 6 h / 100 °C / Inert atmosphere
3: N-ethyl-N,N-diisopropylamine / dimethyl sulfoxide / 48 h / 130 °C / Sealed tube
With
tetrakis(triphenylphosphine) palladium(0); dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; potassium carbonate; N-ethyl-N,N-diisopropylamine;
In
1,4-dioxane; water; dimethyl sulfoxide; acetonitrile;
2: |Suzuki Coupling;
|
|
|
Multi-step reaction with 3 steps
1: potassium acetate; dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 / 1,4-dioxane / 15 h / 110 °C / Inert atmosphere
2: tetrakis(triphenylphosphine) palladium(0); potassium carbonate / acetonitrile / 6 h / 100 °C
3: N-ethyl-N,N-diisopropylamine / dimethyl sulfoxide / 48 h / 130 °C / Sealed tube
With
tetrakis(triphenylphosphine) palladium(0); dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; potassium carbonate; N-ethyl-N,N-diisopropylamine;
In
1,4-dioxane; dimethyl sulfoxide; acetonitrile;
2: |Suzuki Coupling;
|
|
|
Multi-step reaction with 3 steps
1: potassium acetate; dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 / 1,4-dioxane / 15 h / 110 °C / Inert atmosphere
2: tetrakis(triphenylphosphine) palladium(0); potassium carbonate / acetonitrile; water / 6 h / 100 °C / Inert atmosphere
3: N-ethyl-N,N-diisopropylamine / dimethyl sulfoxide / 48 h / 130 °C / Sealed tube
With
tetrakis(triphenylphosphine) palladium(0); dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; potassium carbonate; N-ethyl-N,N-diisopropylamine;
In
1,4-dioxane; water; dimethyl sulfoxide; acetonitrile;
|
|
|
Multi-step reaction with 3 steps
1: potassium acetate; dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 / 1,4-dioxane / 15 h / 110 °C / Inert atmosphere
2: tetrakis(triphenylphosphine) palladium(0); potassium carbonate / water; acetonitrile / 6 h / 100 °C / Inert atmosphere
3: N-ethyl-N,N-diisopropylamine / dimethyl sulfoxide / 48 h / 130 °C / Inert atmosphere
With
tetrakis(triphenylphosphine) palladium(0); dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; potassium carbonate; N-ethyl-N,N-diisopropylamine;
In
1,4-dioxane; water; dimethyl sulfoxide; acetonitrile;
2: |Suzuki Coupling;
|
|
|
Multi-step reaction with 3 steps
1.1: potassium acetate / acetonitrile / 0.5 h / Reflux
1.2: 4 h / Reflux
2.1: potassium acetate; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / acetonitrile; water / 3 h / Reflux
3.1: hydrogenchloride / 1-Propyl acetate; propan-1-ol / 15 h / 75 °C
With
hydrogenchloride; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
propan-1-ol; 1-Propyl acetate; water; acetonitrile;
|
|
|
Multi-step reaction with 3 steps
1: dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate / 1,4-dioxane / 15 h / 110 °C / Inert atmosphere
2: tetrakis(triphenylphosphine) palladium(0); potassium carbonate / acetonitrile; water / 6 h / 100 °C
3: N-ethyl-N,N-diisopropylamine / dimethyl sulfoxide / 48 h / 130 °C / Sealed tube
With
tetrakis(triphenylphosphine) palladium(0); dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; potassium carbonate; N-ethyl-N,N-diisopropylamine;
In
1,4-dioxane; water; dimethyl sulfoxide; acetonitrile;
|
-
![2-(2,8-dimethylimidazo[1,2-b]pyridazin-6-yl)-7-fluoro-pyrido[1,2-a]pyrimidin-4-one](/upload/2023/11/101a0b16-3bcb-4d04-abb3-fc15c125134f.png)
-
2-(2,8-dimethylimidazo[1,2-b]pyridazin-6-yl)-7-fluoro-pyrido[1,2-a]pyrimidin-4-one

-
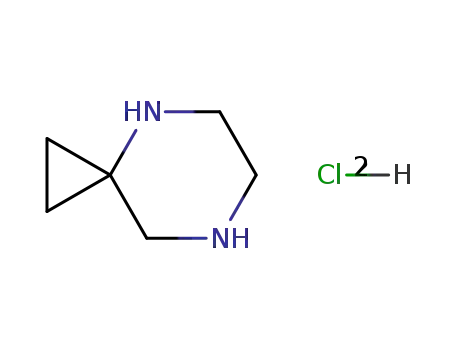
-
145122-56-1
4,7-diazaspiro<2,5>octane dihydrochloride

-
![7-(4,7-diazaspiro[2.5]octan-7-yl)-2-(2,8-dimethylimidazo[l,2- b]pyridazin-6-yl)-4H-pyrido[l,2-a]pyrimidin-4-one](/upload/2023/11/4843d8bb-6708-4bb2-816a-d69c38a758e8.png)
-
1825352-65-5
7-(4,7-diazaspiro[2.5]octan-7-yl)-2-(2,8-dimethylimidazo[l,2- b]pyridazin-6-yl)-4H-pyrido[l,2-a]pyrimidin-4-one
| Conditions | Yield |
|---|---|
|
With
N-ethyl-N,N-diisopropylamine;
In
dimethyl sulfoxide;
at 130 ℃;
for 48h;
Sealed tube;
|
18% |
|
With
N-ethyl-N,N-diisopropylamine;
In
dimethyl sulfoxide;
at 130 ℃;
for 48h;
Sealed tube;
|
18% |
|
With
N-ethyl-N,N-diisopropylamine;
In
dimethyl sulfoxide;
at 130 ℃;
for 48h;
Sealed tube;
|
18% |
|
With
N-ethyl-N,N-diisopropylamine;
In
dimethyl sulfoxide;
at 130 ℃;
for 48h;
Inert atmosphere;
|
18% |
|
With
N-ethyl-N,N-diisopropylamine;
In
dimethyl sulfoxide;
at 130 ℃;
for 48h;
Sealed tube;
|
18% |
1825352-65-5 Upstream products
-
19064-64-3
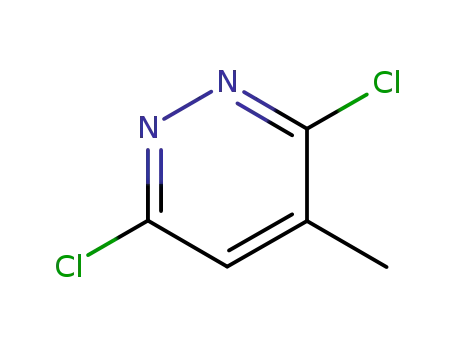
3,6-dichloro-4-methylpyridazine
-
17412-23-6
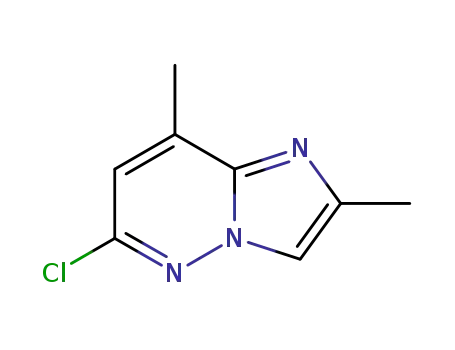
6-chloro-2,8-dimethyl-imidazo[1,2-b]pyridazine
-
1825352-86-0
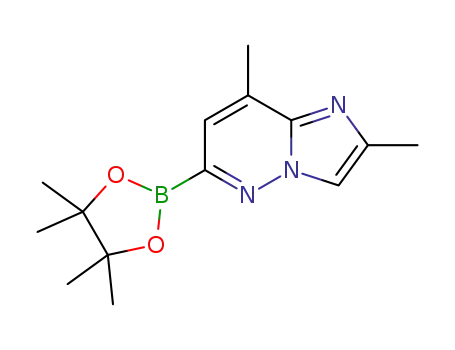
2,8-dimethyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)imidazo[1,2-b]pyridazine
-
145122-56-1

4,7-diazaspiro<2,5>octane dihydrochloride
Relevant Products
-
Bremelanotide acetate
CAS:1607799-13-2
-
BRETAZENIL
CAS:84379-13-5
-
Albendazole
CAS:54965-21-8
-
Bemotrizinol
CAS:187393-00-6