334-50-9
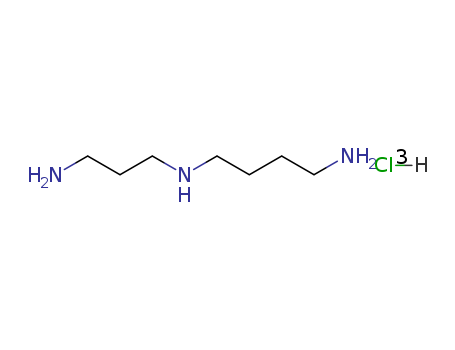
- Product Name:Spermidine Trihydrochloride
- Molecular Formula:C7H19 N3 . 3 Cl H
- Purity:99%
- Molecular Weight:254.631
Product Details;
CasNo: 334-50-9
Molecular Formula: C7H19 N3 . 3 Cl H
Appearance: white crystalline powder
Factory Supply Wholesale Spermidine Trihydrochloride 334-50-9 Fast Shipping
- Molecular Formula:C7H19 N3 . 3 Cl H
- Molecular Weight:254.631
- Appearance/Colour:white crystalline powder
- Vapor Pressure:0.0269mmHg at 25°C
- Melting Point:257-259 °C(lit.)
- Boiling Point:246.6 °C at 760 mmHg
- Flash Point:118.1 °C
- PSA:64.07000
- Density:0.906 g/cm3
- LogP:3.86120
SPERMIDINE TRIHYDROCHLORIDE(Cas 334-50-9) Usage
|
Description |
Spermidine trihydrochloride is a polyamine that inhibits neuronal nitric oxide synthase (nNOS) and binds and precipitates DNA. It may be used to purify DNA binding proteins. Additionally, spermidine stimulates T4 polynucleotide kinase activity. It is involved in growth, development, and the stress response in plants. |
|
Chemical Properties |
Spermidine trihydrochloride appears as white to off-white crystalline powder, which is soluble in water(100mg/ml-clear, colorless solution), very hygroscopic, and air-sensitive. |
|
Uses |
Spermidine Trihydrochloride is a NOS1 inhibitor and NMDA and T4 activator. Polyamine that plays an important role in the regulation of cellular proliferation and differentiation. It was in a structural and functional study of polyamines, where potassium and sodium ions were found to promote different effects when binding with polyamines. Spermidine trihydrochloride has been used in fourier transform infrared spectroscopy (FTIR)characterization and in zeta-potential measurements. |
|
General Description |
Spermidine trihydrochloride is the hydrochloric acid neutralized salt of spermidine. Spermidine is a polyamine and a trivalent organic cation. It is a natural polyamine that stimulates cytoprotective macroautophagy/autophagy. External supplementation of spermidine extends lifespan and health span across species, including in yeast, nematodes, flies and mice. Spermidine trihydrochloride is a more stable form because spermidine is very air sensitive. |
|
Biological Activity |
Binds to the polyamine modulatory site of the NMDA receptor and has been described as an agonist based on its ability to enhance the binding of [ 3 H]-MK801. Spermidine trihydrochloride is an aliphatic polyamine. Its crystalline structure has been studied. It has been reported to preserve the nuclei and prevent proteolytic degradation of histones during the macronuclei isolation from Tetrahymena pyriformis. |
|
Purification Methods |
Recrystallise the salt from dry 3% HCl in ethanol and adding dry Et2O if necessary. Filter it off rapidly and dry it in a vacuum desiccator. Alternatively centrifuge the crystals off, wash them with dry Et2O and dry them in a vacuum. [Beilstein 4 IV 1300.] |
InChI:InChI=1/C7H19N3.3ClH/c8-4-1-2-6-10-7-3-5-9;;;/h10H,1-9H2;3*1H
334-50-9 Relevant articles
Spermidinium closo-dodecaborate-encapsulating liposomes as efficient boron delivery vehicles for neutron capture therapy
Tachikawa, Shoji,Miyoshi, Tatsuro,Koganei, Hayato,El-Zaria, Mohamed E.,Vias, Clara,Suzuki, Minoru,Ono, Koji,Nakamura, Hiroyuki
, p. 12325 - 12328 (2014)
closo-Dodecaborate-encapsulating liposom...
Synthesis process of spermidine and intermediate thereof
-
Paragraph 0047-0053, (2021/10/27)
The invention provides a synthesis proce...
Method for synthesizing spermidine hydrochloride
-
Paragraph 0024-0025; 0034; 0036; 0037; 0039, (2020/07/13)
The invention relates to a method for sy...
Synthesis of 13C-dilabeled 4-coumaroylspermidines
Geneste, Herve,Hesse, Manfred
, p. 15199 - 15214 (2007/10/03)
Five 13C-dilabeled constitution isomers ...
334-50-9 Process route
-
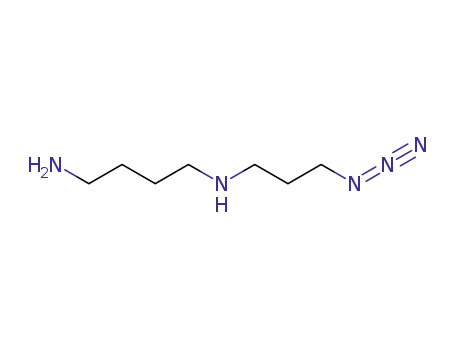
-
N-(3-azidopropyl)-1,4-diaminobutane

-
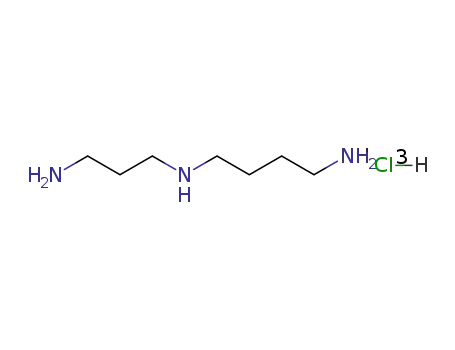
-
334-50-9,35408-56-1
spermidine trihydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; hydrogen;
palladium on activated charcoal;
In
ethanol;
for 18h;
under 3102.9 Torr;
Ambient temperature;
|
91% |
-
![tert-butyl {4-{[3-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)propyl]amino}butyl}carbamate](/upload/2023/11/358772c9-29ae-4e8c-8888-f74057d4c240.png)
-
191277-07-3
tert-butyl {4-{[3-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)propyl]amino}butyl}carbamate

-

-
334-50-9,35408-56-1
spermidine trihydrochloride
| Conditions | Yield |
|---|---|
|
tert-butyl {4-{[3-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)propyl]amino}butyl}carbamate;
With
hydrazine hydrate;
In
ethanol;
for 4h;
Reflux;
Large scale;
With
hydrogenchloride;
In
ethanol; water;
Reagent/catalyst;
Reflux;
Large scale;
|
78.9% |
334-50-9 Upstream products
-
130750-16-2
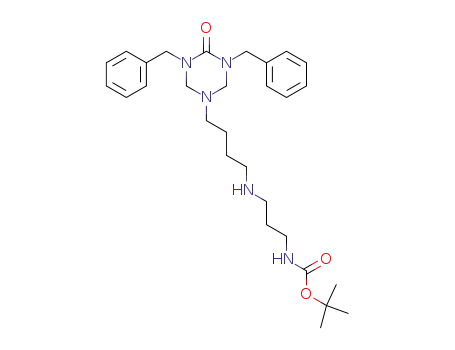
{3-[4-(3,5-Dibenzyl-4-oxo-[1,3,5]triazinan-1-yl)-butylamino]-propyl}-carbamic acid tert-butyl ester
-
81065-67-0
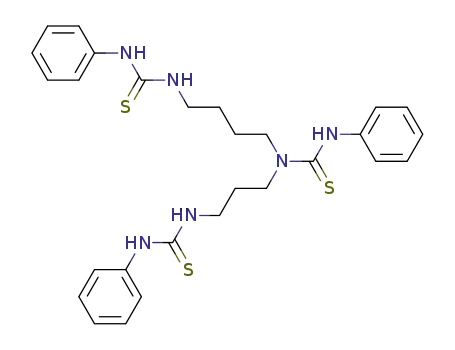
N,N',N''-tris(phenylaminothiocarbonyl)-N-(3-aminopropyl)-1,4-diaminobutane
-
182576-24-5
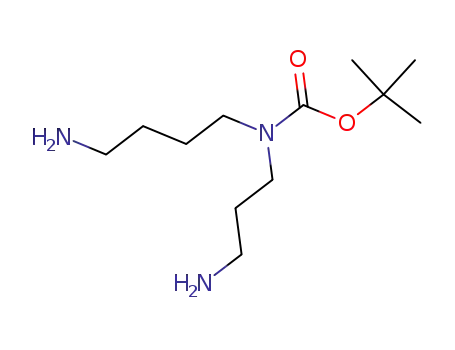
tert butyl (4 aminobutyl)(3-aminopropyl)carbamate
-
124-20-9
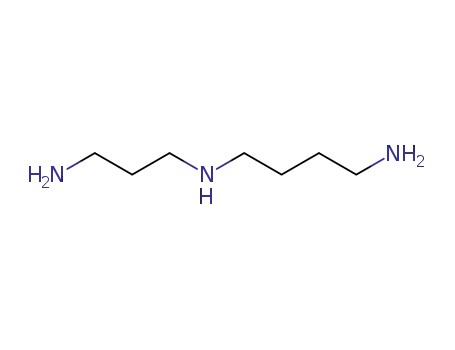
N-(3-aminopropyl)-1,4-diaminobutane
334-50-9 Downstream products
-
131086-79-8
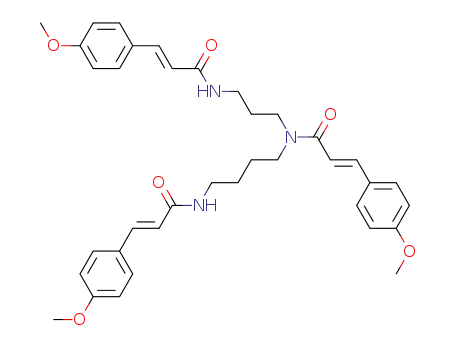
3-(4-methoxy-phenyl)-N-{4-[3-(4-methoxy-phenyl)-acryloylamino]-butyl}-N-{3-[3-(4-methoxy-phenyl)-acryloylamino]-propyl}-acrylamide
-
2465-97-6
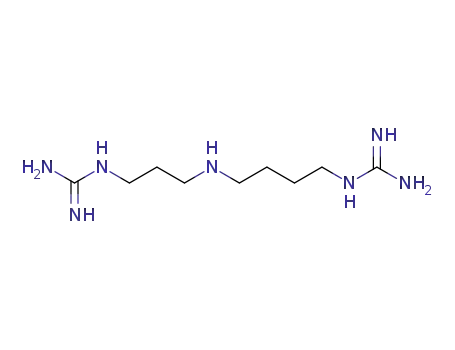
N-(4-guanidinylbutyl)-N-(3-guanidinylpropyl)amine
-
131086-78-7
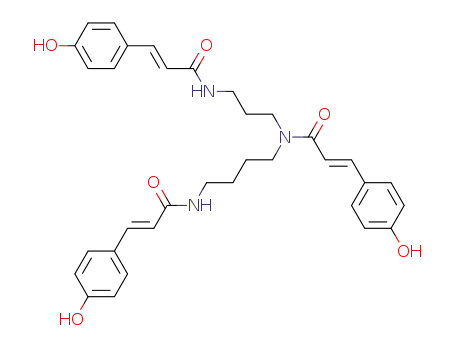
N1,N5,N10-tri-<(E)-p-coumaroyl>-spermidine
-
124-20-9

N-(3-aminopropyl)-1,4-diaminobutane
Relevant Products
-
Toremifene Citrate
CAS:89778-27-8
-
PRO-Xylane
CAS:439685-79-7
-
Tesofensine powder
CAS:402856-42-2