564-35-2
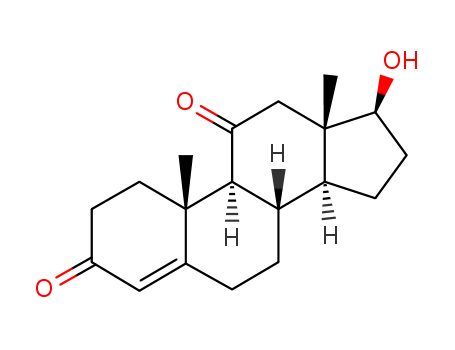
- Product Name:11-KETOTESTOSTERONE
- Molecular Formula:C19H26O3
- Purity:99%
- Molecular Weight:302.414
Product Details;
CasNo: 564-35-2
Molecular Formula: C19H26O3
Chinese Manufacturer Supply Top Purity 11-KETOTESTOSTERONE 564-35-2 Cheapest Price
- Molecular Formula:C19H26O3
- Molecular Weight:302.414
- Vapor Pressure:4.3E-11mmHg at 25°C
- Melting Point:183-184 °C(Solv: acetone (67-64-1); hexane (110-54-3))
- Refractive Index:1.569
- Boiling Point:476.791 °C at 760 mmHg
- PKA:14.79±0.60(Predicted)
- Flash Point:256.265 °C
- PSA:54.37000
- Density:1.191 g/cm3
- LogP:3.05820
11-KETOTESTOSTERONE(Cas 564-35-2) Usage
|
Description |
11-keto Testosterone (CRM) (Item No. 9002654) is a certified reference material that is structurally categorized as an androgen. It is a significant hormone in certain fish, regulating the development of sexual development and behavior. 11-keto Testosterone binds the stickleback kidney androgen receptor (EC50 = 43 nM). 11-keto Testosterone, at 10 nM, significantly activates the human androgen receptor expressed in zebrafish liver cells. This product is intended for research and forensic applications. |
|
Uses |
A metabolite of Adrenosterone. |
|
Biochem/physiol Actions |
11-Ketotestosterone is a minor androgen in humans, but a major androgen in fish. |
InChI:InChI=1/C19H26O3/c1-18-8-7-12(20)9-11(18)3-4-13-14-5-6-16(22)19(14,2)10-15(21)17(13)18/h9,13-14,16-17,22H,3-8,10H2,1-2H3/t13-,14-,16-,17+,18-,19-/m0/s1
564-35-2 Relevant articles
Structure determination of microbial metabolites by the crystalline sponge method
Inokuma, Yasuhide,Ukegawa, Tomoya,Hoshino, Manabu,Fujita, Makoto
, p. 3910 - 3913 (2016/06/09)
The structures of metabolites produced i...
A simple two-step method for the conversion of [3H]cortisol to [3H]- 11-ketotestosterone
Lokman, P. Mark,Irwin, Jacob L.,Blackwell, Leonard F.,Davie, Peter S.,Thomas, Mervyn,Young, Graham
, p. 655 - 658 (2007/10/03)
Despite the existence of several protoco...
Synthesis of 11-substituted androstenediones and testosterones as human decidual cell growth inhibitors
Zhao, Qinjian,Li, Zhensu
, p. 190 - 195 (2007/10/02)
11α-Hydroxytestosterone (1a), 11β-hydrox...
INTRAMOLECULAR DIELS-ALDER REACTION WITH FURAN-DIENE. TOTAL SYNTHESIS OF (+/-)-11-KETOTESTOSTERONE AND (+/-)-ADRENOSTERONE.
Royen, Luc A. Van,Mijngheer, Roelant,Clercq, Pierre J. De
, p. 4667 - 4680 (2007/10/02)
A novel D BCD ABCD route to 11-keto st...
564-35-2 Process route
-
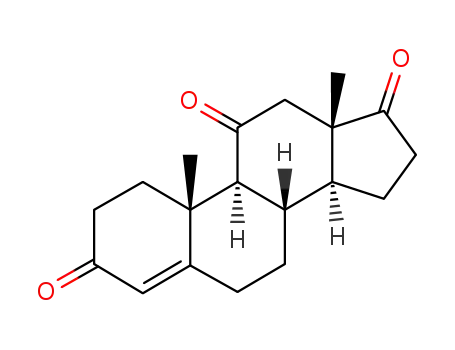
-
382-45-6
adrenosterone

-
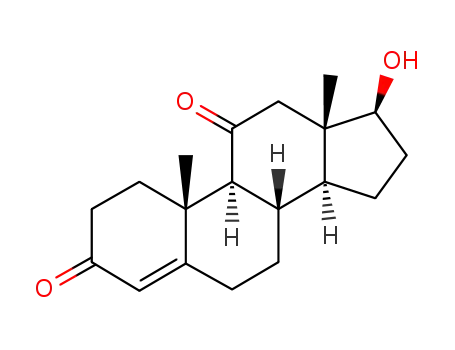
-
564-35-2
11-ketotestosterone
| Conditions | Yield |
|---|---|
|
With
sodium tetrahydroborate;
In
pyridine; methanol;
|
67% |
|
With
tris-buffer; 1,4-dihydronicotinamide adenine dinucleotide; 17β-hydroxysteroid dehydrogenase;
In
ethylene glycol;
for 1h;
Ambient temperature;
|
15% |
|
With
sodium tetrahydroborate;
|
|
|
mit Hilfe von Saccharomyces cerevisiae;
|
|
|
With
sodium tetrahydroborate;
In
ethanol;
|
|
|
With
baker's yeast;
|
-
![(8S,9S,10R,13S,14S,17S)-10,13-Dimethyl-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-3,11,17-triol](/upload/2023/11/e53c2409-58a2-42a8-a1e9-d0c0edef2411.png)
-
81176-76-3
(8S,9S,10R,13S,14S,17S)-10,13-Dimethyl-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-3,11,17-triol

-

-
382-45-6
adrenosterone

-

-
564-35-2
11-ketotestosterone
| Conditions | Yield |
|---|---|
|
With
4-(N,N-dimethylamino)pyridinium chlorochromate;
In
dichloromethane;
for 4h;
|
49% 3% |
564-35-2 Upstream products
-
382-45-6

adrenosterone
-
1420-71-9
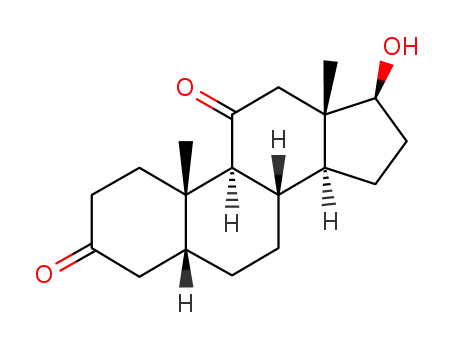
11-keto-5β-dihydrotestosterone
-
6298-21-1
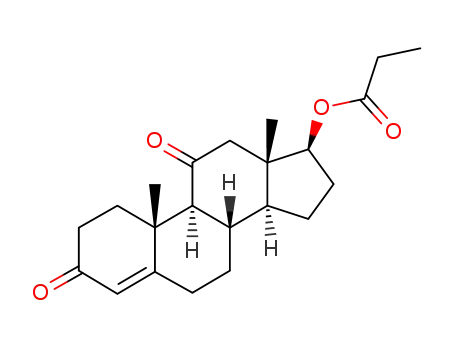
17β-propionyloxy-androst-4-ene-3,11-dione
-
4271-84-5
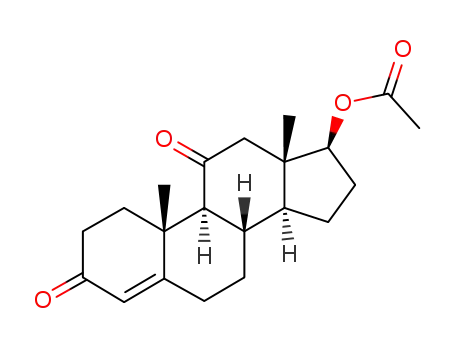
17-acetoxyandrost-4-ene-3,11-dione
564-35-2 Downstream products
-
739-27-5
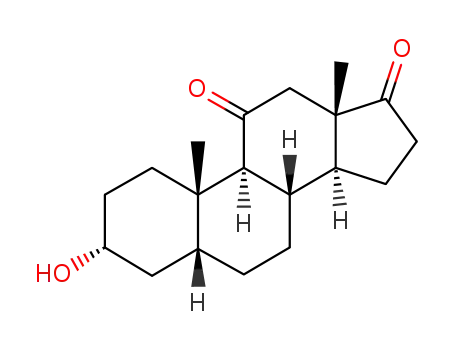
11-ketoetiocholanolone
-
1158-94-7
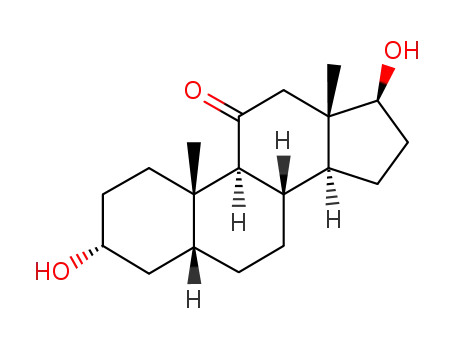
3α,17β-dihydroxy-5β-androstan-11-one
-
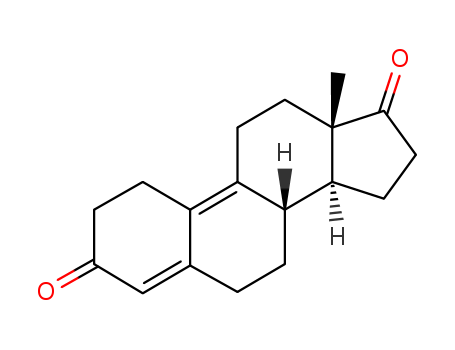
382-45-6
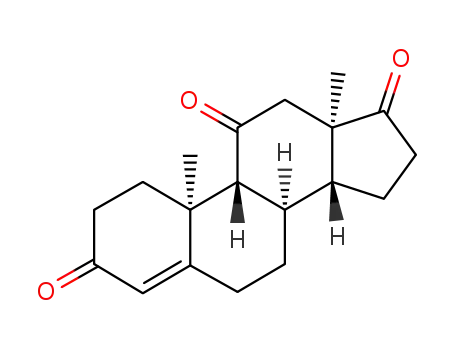
(+/-)-adrenosterone
-
32694-37-4
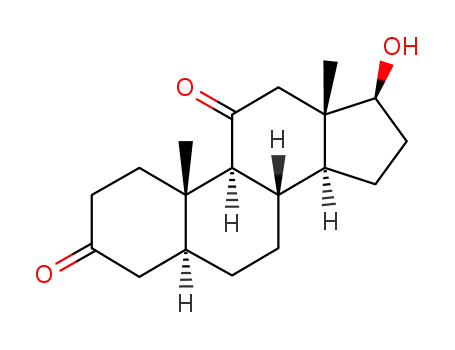
11-ketodihydrotestosterone
Relevant Products
-
Tesofensine powder
CAS:402856-42-2
-
PYRROLOQUINOLINE QUINONE DISODIUM SALT
CAS:122628-50-6
-
Estra-4,9-diene-3,17-dione
CAS:5173-46-6