2697-85-0
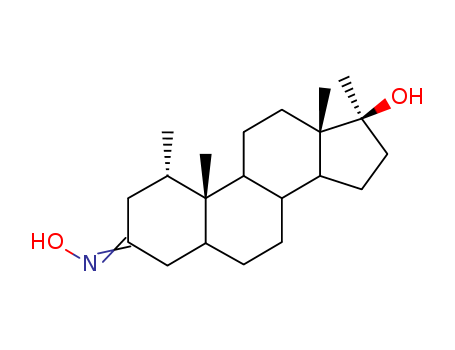
- Product Name:Androst-5-ene-3,7,17-triol
- Molecular Formula:C19H30 O3
- Purity:99%
- Molecular Weight:306.445
Product Details;
CasNo: 2697-85-0
Molecular Formula: C19H30 O3
Buy Quality 99% Pure Androst-5-ene-3,7,17-triol 2697-85-0 Safe Delivery
- Molecular Formula:C19H30 O3
- Molecular Weight:306.445
- Melting Point:235-237℃
- Boiling Point:474.3±45.0 °C(Predicted)
- PKA:14.02±0.70(Predicted)
- PSA:60.69000
- Density:1.19±0.1 g/cm3(Predicted)
- LogP:2.64180
2697-85-0 Relevant articles
The generation of a steroid library using filamentous fungi immobilized in calcium alginate Dedicated to the memory of Professor Sir John W. Cornforth, University of Sussex (1917-2013).
Peart, Patrice C.,Reynolds, William F.,Reese, Paul B.
, p. 16 - 24 (2016/01/25)
Four fungi, namely, Rhizopus oryzae ATCC...
Hydroxylation of DHEA and its analogues by Absidia coerulea AM93. Can an inducible microbial hydroxylase catalyze 7α- and 7β-hydroxylation of 5-ene and 5α-dihydro C19-steroids?
Milecka-Tronina, Natalia,Ko?ek, Teresa,?wizdor, Alina,Panek, Anna
, p. 883 - 891 (2014/01/23)
In this paper we focus on the course of ...
Biotransformation of 3β-hydroxy-5-en-steroids by Mucor silvaticus
Wang, Yanjie,Sun, Dongmei,Chen, Zhibao,Ruan, Hongsheng,Ge, Wenzhong
, p. 168 - 174 (2013/09/12)
The biotransformation of four 3β-hydroxy...
Hydroxylation of DHEA, androstenediol and epiandrosterone by Mortierella isabellina AM212. Evidence indicating that both constitutive and inducible hydroxylases catalyze 7α- as well as 7β-hydroxylations of 5-ene substrates
Kolek, Teresa,Milecka, Natalia,Swizdor, Alina,Panek, Anna,Bialonska, Agata
scheme or table, p. 5414 - 5422 (2011/09/13)
The course of transformation of DHEA, an...
2697-85-0 Process route
-
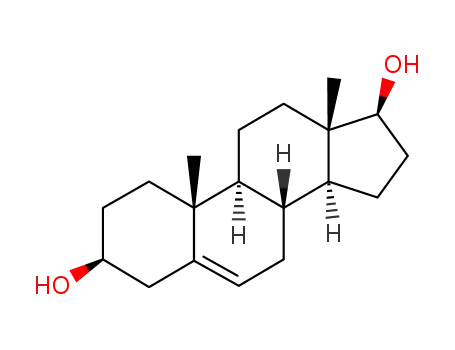
-
521-17-5,1963-03-7,4593-57-1,4593-58-2,14504-94-0,16895-59-3,47122-33-8,64162-67-0,75767-22-5,139973-51-6,151284-19-4
5-androstenediol

-
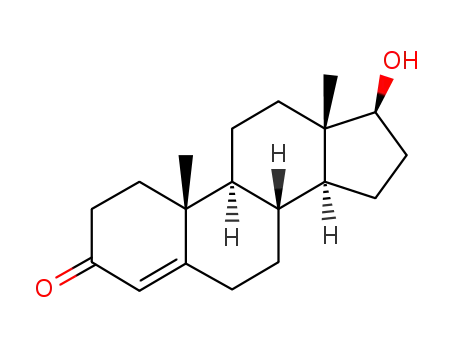
-
58-22-0
testosterone

-
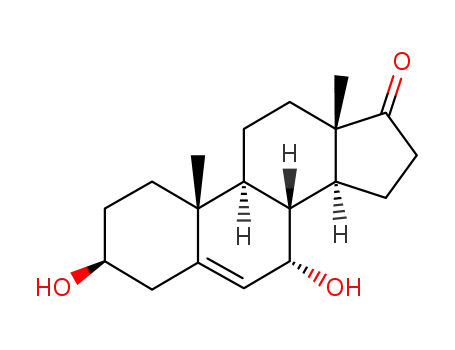
-
2487-48-1,7522-54-5,62357-03-3,53-00-9
3β,7α-dihydroxyandrost-5-ene-17-one

-
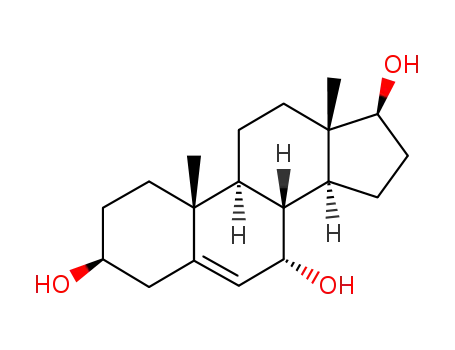
-
2697-85-0,62357-04-4,2226-66-6
Androst-5-ene-3beta,7alpha,17beta-triol

-
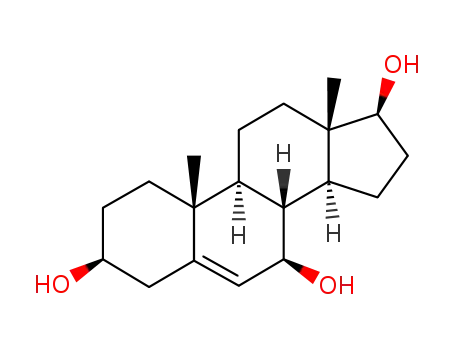
-
2697-85-0
5-androstene-3β,7β,17β-triol
| Conditions | Yield |
|---|---|
|
With
water;
In
ethanol;
for 120h;
Microbiological reaction;
|
-

-
521-17-5,1963-03-7,4593-57-1,4593-58-2,14504-94-0,16895-59-3,47122-33-8,64162-67-0,75767-22-5,139973-51-6,151284-19-4
5-androstenediol

-

-
58-22-0
testosterone

-

-
2487-48-1,7522-54-5,62357-03-3,53-00-9
3β,7α-dihydroxyandrost-5-ene-17-one

-

-
2697-85-0,62357-04-4,2226-66-6
Androst-5-ene-3beta,7alpha,17beta-triol

-

-
2697-85-0
5-androstene-3β,7β,17β-triol
| Conditions | Yield |
|---|---|
|
With
water;
In
ethanol;
for 120h;
Microbiological reaction;
|
2697-85-0 Upstream products
-
53-43-0
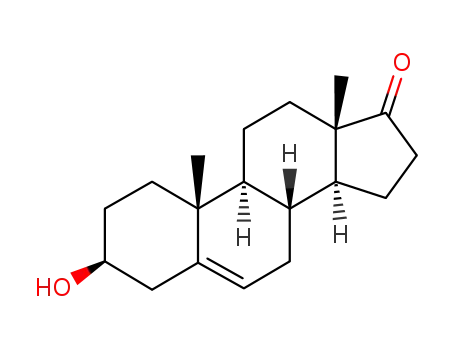
dehydroepiandrosterone
-
2226-65-5
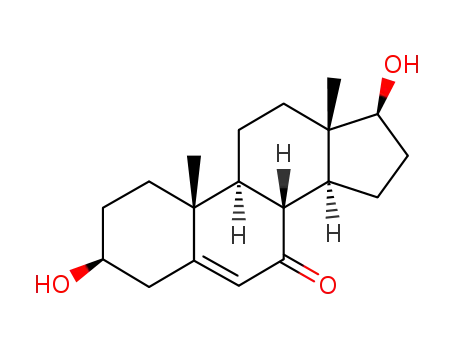
3beta,17beta-Dihydroxyandrost-5-en-7-one
-
13209-60-4
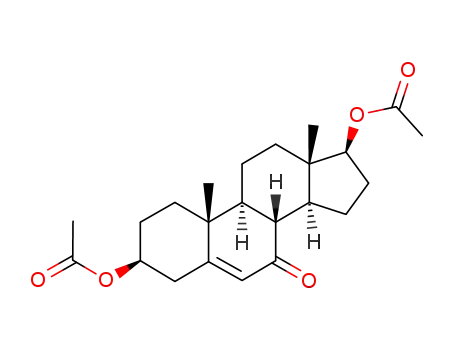
3β, 17β-diacetoxyandrost-5-ene-7-one
-
521-17-5

5-androstenediol
Relevant Products
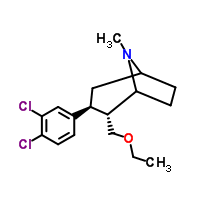
-
Tesofensine powder
CAS:402856-42-2
-
ProMestanolone
CAS:5915-39-9
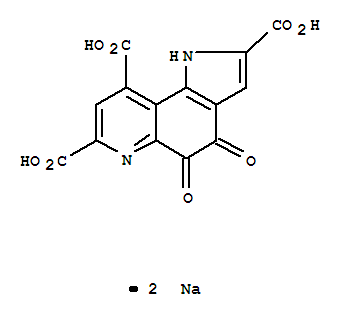
-
PYRROLOQUINOLINE QUINONE DISODIUM SALT
CAS:122628-50-6