56786-63-1
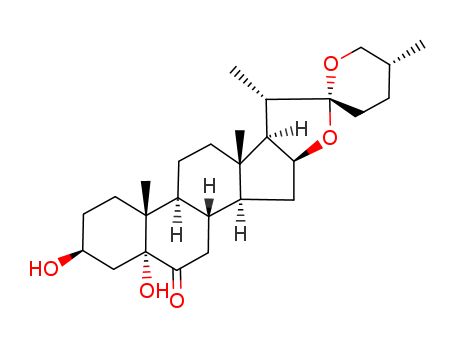
- Product Name:5a-hydroxy Laxogenin
- Molecular Formula:C27H42O5
- Purity:99%
- Molecular Weight:446.627
Product Details;
CasNo: 56786-63-1
Molecular Formula: C27H42O5
Appearance: White or off-white powder
Factory Sells Chinese Manufacturer Supply 5a-hydroxy Laxogenin 56786-63-1 In Bulk Supply
- Molecular Formula:C27H42O5
- Molecular Weight:446.627
- Appearance/Colour:White or off-white powder
- Melting Point:268-270 oC (ethanol )
- Boiling Point:578.4±50.0 °C(Predicted)
- PKA:12.62±0.70(Predicted)
- PSA:75.99000
- Density:1.21±0.1 g/cm3 (20 oC 760 Torr)
- LogP:4.08770
56786-63-1 Relevant articles
Synthesis of new steroidal carbamates with plant-growth-promoting activity: Theoretical and experimental evidence
Pacheco, Daylin Fernández,Ceballos, Leonardo González,Castro, Armando Zaldo,Conde González, Marcos R.,de la Torre, Laura González,Rostgaard, Lia Pérez,Espinoza, Luis,Díaz, Katy,Olea, Andrés F.,García, Yamilet Coll
, p. 1 - 19 (2021/03/01)
A priority of modern agriculture is to u...
In vitro neuroprotective and anti-inflammatory activities of natural and semi-synthetic spirosteroid analogues
García-Pupo, Laura,Zaldo-Castro, Armando,Exarchou, Vassiliki,Tacoronte-Morales, Juan Enrique,Pieters, Luc,Berghe, Wim Vanden,Nu?ez-Figueredo, Yanier,Delgado-Hernández, René
, (2016/08/12)
Two spirosteroid analogues were synthesi...
Novel 5β-hydroxyspirostan-6-ones ecdysteroid antagonists: Synthesis and biological testing
Rivera, Daniel G.,León, Fredy,Coll, Francisco,Davison, Gema P.
, p. 1 - 11 (2007/10/03)
Eight new 5β-hydroxy-spirostan-6-ones be...
The preparation of the spirostanic analogues of brassinolide and castasterone
Robaina Rodriguez, Caridad M.,Teixeira Zullo, Marco Antonio,Queiroz, Helena Mueller,Martins De Azevedo, Mariangela De Burgos,Becerra, Esther Alonso,Manchado, Francisco Coll
, p. 637 - 646 (2007/10/03)
Methods for the preparation of the spiro...
56786-63-1 Process route
-
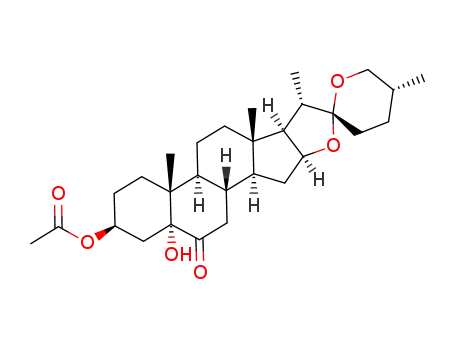
-
5874-17-9
(25R)-3β-acetoxy-5-hydroxy-5α-spirostan-6-one

-
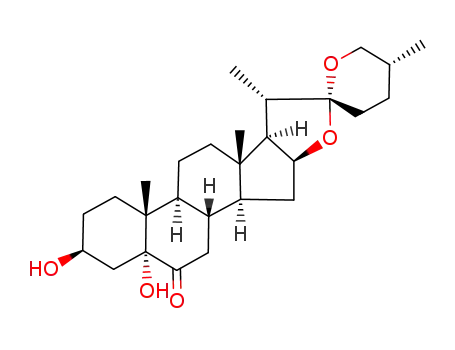
-
56786-63-1
(25R)-3β,5-dihydroxy-5α-spirostan-6-one
| Conditions | Yield |
|---|---|
|
With
sodium hydrogencarbonate;
In
methanol; water;
for 3h;
Reflux;
|
95% |
-
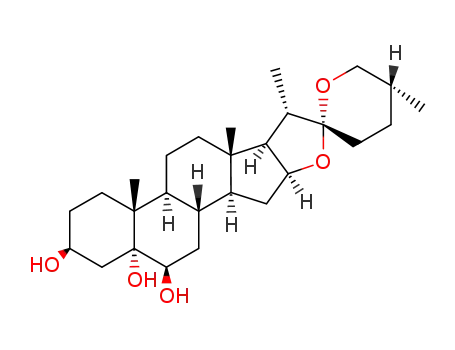
-
56816-69-4
(25R)-spirostan-22α-O-3β,5α,6β-triol

-

-
56786-63-1
(25R)-3β,5-dihydroxy-5α-spirostan-6-one
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
1,4-dioxane; water;
at 20 ℃;
for 2h;
Darkness;
|
83% |
|
Multi-step reaction with 3 steps
1: 84 percent / imidazole / dimethylformamide / 3 h
2: PCC / CH2Cl2 / 0 - 20 °C
3: 7.14 g / TBFA / tetrahydrofuran / 6 h
With
1H-imidazole; pyridinium chlorochromate;
In
tetrahydrofuran; dichloromethane; N,N-dimethyl-formamide;
|
56786-63-1 Upstream products
-
127128-79-4
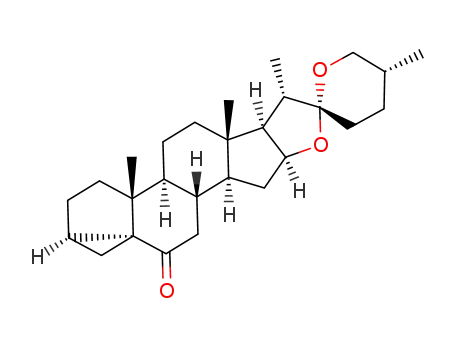
(25R)-3α,5-cyclo-5α-spirostan-6-one
-
876908-18-8
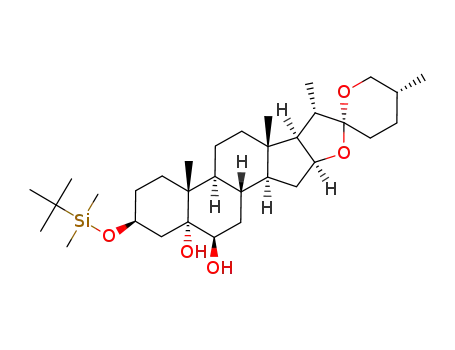
(25R)-3β-(t-butyldimethylsilyloxy)-5α-spirostane-5,6β-diol
-
56816-69-4

(25R)-spirostan-22α-O-3β,5α,6β-triol
-
5874-17-9

(25R)-3β-acetoxy-5-hydroxy-5α-spirostan-6-one
56786-63-1 Downstream products
-
5874-17-9

(25R)-3β-acetoxy-5-hydroxy-5α-spirostan-6-one
-
1373440-75-5
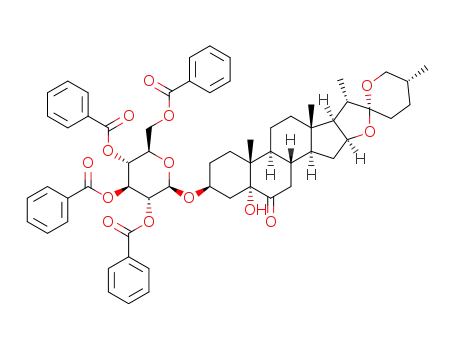
5α-hydroxylaxogenyl 2,3,4,6-tetra-O-benzoyl-β-D-glucopyranoside
-
1373440-86-8
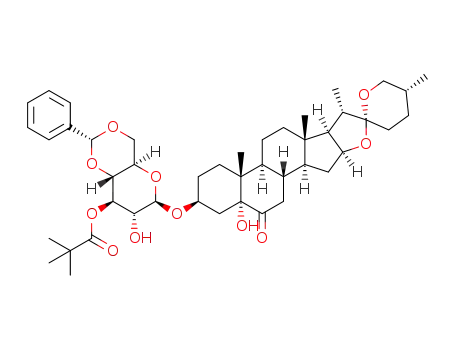
5α-hydroxylaxogenyl 4,6-O-benzylidene-3-O-pivaloyl-β-D-glucopyranoside
-
1373440-84-6
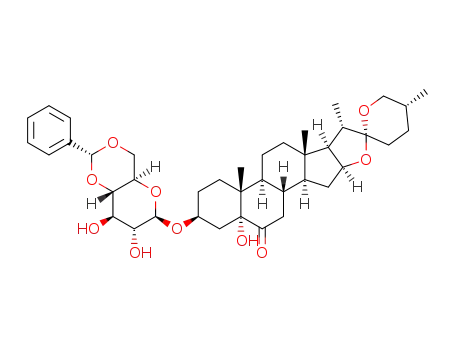
C40H56O10
Relevant Products
-
Tesofensine powder
CAS:402856-42-2
-
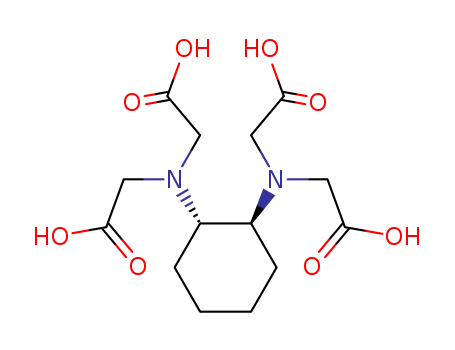
1,2-Cyclohexylenedinitrilotetraacetic acid
CAS:13291-61-7
-
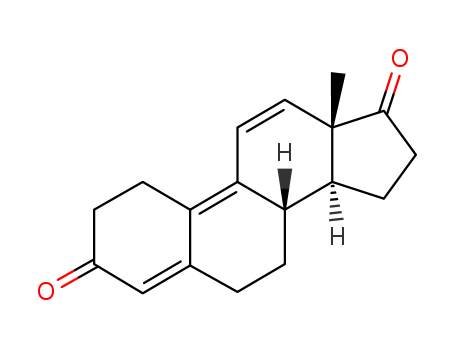
Trendione
CAS:4642-95-9